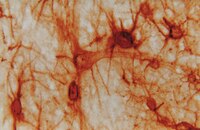
A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia.
Windrem, MS; Schanz, SJ; Morrow, C; Munir, J; Chandler-Militello, D; Wang, S; Goldman, SA
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
16153-61
2014
Show Abstract
Neonatally transplanted human glial progenitor cells (hGPCs) densely engraft and myelinate the hypomyelinated shiverer mouse. We found that, in hGPC-xenografted mice, the human donor cells continue to expand throughout the forebrain, systematically replacing the host murine glia. The differentiation of the donor cells is influenced by the host environment, such that more donor cells differentiated as oligodendrocytes in the hypomyelinated shiverer brain than in myelin wild-types, in which hGPCs were more likely to remain as progenitors. Yet in each recipient, both the number and relative proportion of mouse GPCs fell as a function of time, concomitant with the mitotic expansion and spread of donor hGPCs. By a year after neonatal xenograft, the forebrain GPC populations of implanted mice were largely, and often entirely, of human origin. Thus, neonatally implanted hGPCs outcompeted and ultimately replaced the host population of mouse GPCs, ultimately generating mice with a humanized glial progenitor population. These human glial chimeric mice should permit us to define the specific contributions of glia to a broad variety of neurological disorders, using human cells in vivo. | 25429155
 |
Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination.
Wang, S; Bates, J; Li, X; Schanz, S; Chandler-Militello, D; Levine, C; Maherali, N; Studer, L; Hochedlinger, K; Windrem, M; Goldman, SA
Cell stem cell
12
252-64
2013
Show Abstract
Neonatal engraftment by oligodendrocyte progenitor cells (OPCs) permits the myelination of the congenitally dysmyelinated brain. To establish a potential autologous source of these cells, we developed a strategy by which to differentiate human induced pluripotent stem cells (hiPSCs) into OPCs. From three hiPSC lines, as well as from human embryonic stem cells (hESCs), we generated highly enriched OLIG2(+)/PDGFRα(+)/NKX2.2(+)/SOX10(+) human OPCs, which could be further purified using fluorescence-activated cell sorting. hiPSC OPCs efficiently differentiated into both myelinogenic oligodendrocytes and astrocytes, in vitro and in vivo. Neonatally engrafted hiPSC OPCs robustly myelinated the brains of myelin-deficient shiverer mice and substantially increased their survival. The speed and efficiency of myelination by hiPSC OPCs was higher than that previously observed using fetal-tissue-derived OPCs, and no tumors from these grafts were noted as long as 9 months after transplant. These results suggest the potential utility of hiPSC-derived OPCs in treating disorders of myelin loss. | 23395447
 |
Ultrastructural and immunocytochemical detection of keratins and extracellular matrix proteins in lizard skin cultured in vitro.
Lorenzo Alibardi,Elisabetta Polazzi
Tissue & cell
44
2012
Show Abstract
The present study shows the localization of epidermal and dermal proteins produced in lizard skin cultivated in vitro. Cells from the skin have been cultured for up to one month to detect the expression of keratins, actin, vimentin and extracellular matrix proteins (fibronectin, chondroitin sulphate proteoglycan, elastin and collagen I). Keratinocytes and dermal cells weakly immunoreact for Pan-Cytokeratin but not with the K17-antibody at the beginning of the cell culture when numerous keratin bundles are present in keratinocyte cytoplasm. The dense keratin network disappears after 7-12 days in culture, and K17 becomes detectable in both keratinocytes and mesenchymal cells isolated from the dermis. While most epidermal cells are lost after 2 weeks of in vitro cultivation dermal cells proliferate and form a pellicle of variable thickness made of 3-8 cell layers. The fibroblasts of this dermal equivalent produces an extracellular matrix containing chondroitin sulphate proteoglycan, collagen I, elastic fibers and fibronectin, explaining the attachment of the pellicle to the substratum. The study indicates that after improving keratinocyte survival a skin equivalent for lizard epidermis would be feasible as a useful tool to analyze the influence of the dermis on the process of epidermal differentiation and the control of the shedding cycle in squamates. | 22325741
 |
Pleiotrophin suppression of receptor protein tyrosine phosphatase-β/ζ maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells.
McClain, CR; Sim, FJ; Goldman, SA
The Journal of neuroscience : the official journal of the Society for Neuroscience
32
15066-75
2012
Show Abstract
Oligodendrocyte progenitor cells (OPCs) persist in human white matter, yet the mechanisms by which they are maintained in an undifferentiated state are unknown. Human OPCs differentially express protein tyrosine phosphatase receptor β/ζ (PTPRZ1) and its inhibitory ligand, pleiotrophin, suggesting the maintenance of an autocrine loop by which PTPRZ1 activity is tonically suppressed. PTPRZ1 constitutively promotes the tyrosine dephosphorylation of β-catenin and, thus, β-catenin participation in T cell factor (TCF)-mediated transcription. Using CD140a/PDGFRα-based fluorescence-activated cell sorting to isolate fetal OPCs from the fetal brain at gestational ages 16-22 weeks, we asked whether pleiotrophin modulated the expansion of OPCs and, if so, whether this was effected through the serial engagement of PTPRZ1 and β-catenin-dependent signals, such as TCF-mediated transcription. Lentiviral shRNAi knockdown of PTPRZ1 induced TCF-mediated transcription and substantially augmented GSK3β inhibition-induced TCF-reporter luciferase expression, suggesting dual regulation of β-catenin and the importance of PTPRZ1 as a tonic brake upon TCF-dependent transcription. Pharmacological inhibition of GSK3β triggered substrate detachment and initiated sphere formation, yet had no effect on either proliferation or net cell number. In contrast, pleiotrophin strongly potentiated the proliferation of CD140a(+)-sorted OPCs, as did PTPRZ1 knockdown, which significantly increased the total number of population doublings exhibited by OPCs before mitotic senescence. These observations suggest that pleiotrophin inhibition of PTPRZ1 contributes to the homeostatic self-renewal of OPCs and that this process is mediated by the tonic activation of β-catenin/TCF-dependent transcription. | 23100427
 |
Culture, Immortalization and Characterization of Human Meibomian Gland Epithelial Cells.
Liu S, Hatton MP, Khandelwal P, Sullivan DA
Invest Ophthalmol Vis Sci
2010
Show Abstract
Purpose: Meibomian gland epithelial cells are essential in maintaining the health and integrity of the ocular surface. However, very little is known about their physiological regulation. To advance our understanding of the cellular control mechanisms, we sought to: first, establish a defined culture system for the maintenance of primary epithelial cells from human meibomian glands; and second, to immortalize these cells, thereby developing a preclinical model that could be used to identify factors that regulate cell activity. Methods: Human meibomian glands were removed from lid segments after surgery, enzymatically digested and then dissociated. Isolated epithelial cells were cultured in media with or without serum and/or 3T3 feeder layers. To attempt immortalization, cells were exposed to retroviral human telomerase reverse transcriptase (hTERT) and/or SV40 large T antigen cDNA vectors and antibiotic resistant cells were selected, expanded and subcultured. Analyses for possible biomarkers, cell proliferation and differentiation, lipid-related enzyme gene expression and the cellular response to androgen were performed with biochemical, histological and molecular biological techniques. Results: Our studies demonstrate that it is possible to isolate viable human meibomian gland epithelial cells and to culture them in serum-free media. These cells proliferate, survive through at least a 5th passage and contain neutral lipids. Infection with hTERT immortalized these cells, which accumulate neutral lipids during differentiation, express multiple genes for lipogenic enzymes, respond to androgen, and continue to proliferate. Conclusions: Our results show that human meibomian gland epithelial cells may be isolated, cultured and immortalized. | 20335607
 |
Alterations of bone marrow-derived endothelial progenitor cells following acute pulmonary embolism in mice.
Wan J, Lu LJ, Miao R, Liu J, Xu XX, Yang T, Hu QH, Wang J, Wang C
Exp Biol Med (Maywood)
235
989-98.
2010
Show Abstract
Pulmonary embolism (PE) is a common, lethal, ischemic disease. PE-induced endothelium injury plays a critical role in the pathophysiological consequences of PE. Endothelial progenitor cells (EPCs) can be mobilized from the bone marrow to enter circulation and play important roles in repair of damaged endothelium. However, it is not yet known if EPC mobilization results from PE. The alterations of the quantity and function of bone marrow-derived EPCs were detected in acute pulmonary embolism (APE) events in mice, and the possible role of the endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) pathway in those alterations was explored. APE models were established by injection of autologous thrombi into the right jugular vein of C57BL/6 mice. Mice were divided into sham and experimental groups including one hour (1H), one day (1D) and two day (2D) groups after injection. The results showed that in the APE 1D group, the thrombi were easily found in the large or medium pulmonary vessel. And CD133(+) or CD34(+) cells in bone marrow increased significantly, while CD133(+)/vascular endothelial growth factor receptor 2(+) EPCs decreased. After seven days in culture, the abilities of incorporation into a vascular network, adhesion to fibronectin, migration and proliferation of bone marrow-derived EPCs in the APE 1D group increased significantly. The mRNA and protein expression levels of eNOS in EPCs increased in the APE 1D group. Treatment of EPCs with N(G)-nitro-L-arginine methyl ester inhibited functional alterations induced by APE. The results suggested that APE events stimulate the mobilization of EPCs from bone marrow, and enhance their functions. The eNOS/NO pathway may be involved in this process. | 20660099
 |
Long-term growth suppression of human glioma xenografts by chemoimmunoconjugates of 4-desacetylvinblastine-3-carboxyhydrazide and monoclonal antibody 9.2.27.
Schrappe, M, et al.
Cancer Res., 52: 3838-44 (1992)
1992
Show Abstract
A conjugate of 4-desacetylvinblastine-3-carboxyhydrazide (DAVLBHY) and the glioma-reactive monoclonal antibody (mAb) 9.2.27 induced long-term suppression of tumor growth in athymic nude mice engrafted with U87MG human glioma cells. In vitro, DAVLBHY had the strongest antiproliferative activity (inhibitory concentration at which incorporation of [3H]thymidine is at 50% of untreated control is 2.0 x 10(-9) M) of seven cytotoxic drugs tested and so was chosen for conjugation to mAb 9.2.27, which reacts specifically with the core protein of chondroitin sulfate proteoglycans found in human glioblastomas. After conjugation of DAVLBHY to the carbohydrate residues of mAb 9.2.27 it retained its full binding capacity. For in vivo studies, DAVLBHY and several conjugate derivatives were evaluated by using two dosages of i.v. injections, each starting 2 days after s.c. tumor inoculation. The control tumors reached a volume of nearly 3000 mm3 within 30 days. Tumor growth was delayed by about 20 days with four i.v. injections of 0.5 mg/kg 9.2.27-DAVLBHY, which was slightly superior to the unconjugated drug. Moreover, 9.2.27-DAVLBHY produced a highly significant suppression of growth so that the average tumor volume was only 3% of that observed in untreated controls after 28 days. Four injections of this conjugate at a larger dose, 2.0 mg/kg, prevented recurrence of the tumors for 130 days in all animals tested, thus demonstrating a significant increase in the therapeutic index, since the unconjugated drug provided limited inhibition of tumor growth for only 40 days. The specificity of the antitumor effect was demonstrated in a comparison with the control conjugate, KS1/4-DAVLBHY, which despite partial tumor suppression had only a transient effect. The specific antitumor effect of 9.2.27-DAVLBHY was unexpected, since the target antigen is expressed at a relatively low density (40,000 sites/cell) on U87MG glioma cells. | 1617657
 |
Biosynthetic studies of proteoglycans in human melanoma cells with a monoclonal antibody to a core glycoprotein of chondroitin sulfate proteoglycans.
Bumol, T F, et al.
J. Biol. Chem., 259: 12733-41 (1984)
1984
Show Abstract
Monoclonal antibody (Mab) 9.2.27 was utilized in a combination of biosynthetic and biochemical investigations as an immunological probe for the study of chondroitin sulfate proteoglycans (CSP) in human melanoma cells. Pulse-chase and long-term intrinsic labeling immunoprecipitation experiments combined with the biosynthetic inhibitors monensin, cycloheximide, and paranitrophenol-beta-D-xyloside all suggest that Mab 9.2.27 recognizes a set of glycoprotein molecules ranging to a 250-kDa glycoprotein which serves as the core glycoprotein for CSP in human melanoma cells. Peptide maps comparing the 250-kDa and CSP molecule verify that the 250-kDa glycoprotein is the CSP core protein in human melanoma cells. Further studies document that the CSP released by melanoma cells and recognized by Mab 9.2.27 contains (2-acetamido-2-deoxy-3-O-(beta-D-gluco-4-enepyranosyluronic acid)-beta-4-O-sulfo-D-galactose and 2-acetamido-2-deoxy-3-O-(beta-D-gluco-4-enepyranosyluronic acid)-beta-6-O-sulfo-D-galactose saccharides and this CSP can interact with hyaluronic acid-Sepharose. Topographical studies indicate that this CSP has pericellular punctuated distribution on the melanoma cell surface and may play a role in cell-substrate interactions in the biology of metastatic human melanoma. | 6386801
 |
Production and characterization of monoclonal antibody to a melanoma specific glycoprotein.
Morgan, A C, et al.
Hybridoma, 1: 27-36 (1981)
1981
Show Abstract
An immunogen consisting of a 4M urea extract derived from human melanoma cells (M14), that was devoid of HLA-A,B,C, HLA-DR antigens and fibronectin was adsorbed to lens culinaris lectin-Sepharose 4B and used to immunize mice for production of monoclonal antibody to a melanoma-specific glycoprotein. Screening for hybridomas secreting antibodies to melanoma associated antigens was facilitated by use of a solid phase target antigen of chemically defined medium of melanoma cells (CDM). Use of these procedures allowed us to select 40 hybridomas secreting antibody which recognized determinants on melanoma cells not found on lymphoid cells. Further characterization of one of these hybridomas, 9.2.27, indicated that the antibody it secreted recognized a 240K dalton glycoprotein found on all melanoma cell lines tested but not on carcinoma, lymphoid, or fibroblastoid cultures. These results demonstrate the utility of soluble antigen preparations devoid of strongly immunogenic non tumor-specific molecules in the elicitation of tumor specific antibody. Preliminary results suggest that immunogens of this kind are superior to intact melanoma cells for production of tumor specific hybridomas. | 6208119
 |