476275 Sigma-AldrichMyricetin - CAS 529-44-2 - Calbiochem
A cell-permeable flavanoid that displays anti-inflammatory, anti-diabetic and anti-cancer properties.
More>> A cell-permeable flavanoid that displays anti-inflammatory, anti-diabetic and anti-cancer properties. Less<<Sinónimos: 3,3ʹ,4ʹ,5,5ʹ,7-Hexahydroxyflavone, Hsp70 Inhibitor II
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
| 529-44-2 | C₁₅H₁₀O₈ |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 476275-25MG |
|
Ampolla de plást. | 25 mg |
|
— |
| Product Information | |
|---|---|
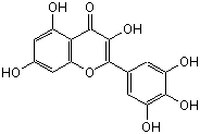
| CAS number | 529-44-2 |
| Form | Yellowish brown solid |
| Hill Formula | C₁₅H₁₀O₈ |
| Chemical formula | C₁₅H₁₀O₈ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 476275-25MG | 04055977184471 |
Documentation
Myricetin - CAS 529-44-2 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Myricetin - CAS 529-44-2 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 476275 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Lee, K.W., et al. 2007. Carcinogenesis 28, 1918. Holder, S., et al. 2007. Mol. Cancer Ther. 6, 163. Strobel, P., et al. 2005. Biochem. J. 386, 471. Ko, C.H., et al. 2005. Mol. Cancer Ther. 4, 281. Ko, C.H., et al. 2005. Biochem. Pharmacol. 69, 913. Ko, W.C., et al. 2004. Biochem. Pharmacol. 68, 2087. Walker, E.H., et al. 2000. Mol. Cell 6, 909. Agullo, G., et al. 1997. Biochem. Pharmacol. 53, 1649. Hagiwara, M., et al. 1988. Biochem. Pharmacol. 37, 2987. |