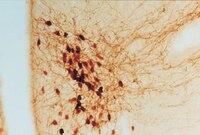
Genetic labeling reveals novel cellular targets of schizophrenia susceptibility gene: distribution of GABA and non-GABA ErbB4-positive cells in adult mouse brain.
Bean, JC; Lin, TW; Sathyamurthy, A; Liu, F; Yin, DM; Xiong, WC; Mei, L
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
13549-66
2014
Mostrar resumen
Neuregulin 1 (NRG1) and its receptor ErbB4 are schizophrenia risk genes. NRG1-ErbB4 signaling plays a critical role in neural development and regulates neurotransmission and synaptic plasticity. Nevertheless, its cellular targets remain controversial. ErbB4 was thought to express in excitatory neurons, although recent studies disputed this view. Using mice that express a fluorescent protein under the promoter of the ErbB4 gene, we determined in what cells ErbB4 is expressed and their identity. ErbB4 was widely expressed in the mouse brain, being highest in amygdala and cortex. Almost all ErbB4-positive cells were GABAergic in cortex, hippocampus, basal ganglia, and most of amygdala in neonatal and adult mice, suggesting GABAergic transmission as a major target of NRG1-ErbB4 signaling in these regions. Non-GABAergic, ErbB4-positive cells were present in thalamus, hypothalamus, midbrain, and hindbrain. In particular, ErbB4 is expressed in serotoninergic neurons of raphe nuclei but not in norepinephrinergic neurons of the locus ceruleus. In hypothalamus, ErbB4 is present in neurons that express oxytocin. Finally, ErbB4 is expressed in a group of cells in the subcortical areas that are positive for S100 calcium binding protein β. These results identify novel cellular targets of NRG1-ErbB4 signaling. | Immunohistochemistry | Mouse | 25274830
 |
Paternal deprivation alters play-fighting, serum corticosterone and the expression of hypothalamic vasopressin and oxytocin in juvenile male mandarin voles.
Jianli Wang,Fadao Tai,Xingfu Yan,Peng Yu
Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology
198
2011
Mostrar resumen
Although early paternal deprivation significantly affects offspring behavioral and neuroendocrine development, the link between paternal deprivation and social play behavior remains unclear. Mandarin voles (Microtus mandarinus) are socially monogamous and display bi-paternal care. The present study examined the development of social play in juvenile male mandarin voles and the paternal influence on play-fighting, vasopressin- and oxytocin-immunoreactive neurons and serum corticosterone and testosterone levels. The results show that social play was more pronounced during postnatal days 28-35, differing from the ontogenetic pattern of other forms of social behavior. On postnatal day 35, the peak in play-fighting activity, paternal deprivation reduced boxing/wrestling levels and vasopressin-immunoreactive neurons in the anterior hypothalamus and oxytocin-immunoreactive neurons in the paraventricular nucleus, but increased vasopressin-immunoreactive neurons in the paraventricular nucleus and corticosterone levels. These results suggest that mandarin voles engage in social play according to an inverted U-shaped curve in ontogeny, and paternal deprivation influences the development of offspring play-fighting; hypothalamic vasopressin, oxytocin and serum corticosterone may play a modulatory role in the alteration of play-fighting elicited by paternal deprivation; decreased play-fighting may correlate with depressed vasopressin levels in the anterior hypothalamus. | | | 22926316
 |
The nonpeptide oxytocin receptor agonist WAY 267,464: receptor-binding profile, prosocial effects and distribution of c-Fos expression in adolescent rats.
Hicks, C; Jorgensen, W; Brown, C; Fardell, J; Koehbach, J; Gruber, CW; Kassiou, M; Hunt, GE; McGregor, IS
Journal of neuroendocrinology
24
1012-29
2011
Mostrar resumen
Previous research suggests that the nonpeptide oxytocin receptor (OTR) agonist WAY 267,464 may only partly mimic the effects of oxytocin in rodents. The present study further explored these differences and related them to OTR and vasopressin 1a receptor (V(1a) R) pharmacology and regional patterns of c-Fos expression. Binding data for WAY 267,464 and oxytocin were obtained by displacement binding assays on cellular membranes, while functional receptor data were generated by luciferase reporter assays. For behavioural testing, adolescent rats were tested in a social preference paradigm, the elevated plus-maze (EPM) and for locomotor activity changes following WAY 267,464 (10 and 100 mg/kg, i.p.) or oxytocin (0.1 and 1 mg/kg, i.p.). The higher doses were also examined for their effects on regional c-Fos expression. Results showed that WAY 267,464 had higher affinity (K(i) ) at the V(1a) R than the OTR (113 versus 978 nm). However, it had no functional response at the V(1a) R and only a weak functional effect (EC(50) ) at the OTR (881 nm). This suggests WAY 267,464 is an OTR agonist with weak affinity and a possible V(1a) R antagonist. Oxytocin showed high binding at the OTR (1.0 nm) and V(1a) R (503 nm), with a functional EC(50) of 9.0 and 59.7 nm, respectively, indicating it is a potent OTR agonist and full V(1a) R agonist. WAY 267,464 (100 mg/kg), but not oxytocin, significantly increased the proportion of time spent with a live rat, over a dummy rat, in the social preference test. Neither compound affected EPM behaviour, whereas the higher doses of WAY 267,464 and oxytocin suppressed locomotor activity. WAY 267,464 and oxytocin produced similar c-Fos expression in the paraventricular hypothalamic nucleus, central amygdala, lateral parabrachial nucleus and nucleus of the solitary tract, suggesting a commonality of action at the OTR with the differential doses employed. However, WAY 267,464 caused greater c-Fos expression in the medial amygdala and the supraoptic nucleus than oxytocin, and lesser effects in the locus coeruleus. Overall, our results confirm the differential effects of WAY 267,464 and oxytocin and suggest that this may reflect contrasting actions of WAY 267,464 and oxytocin at the V(1a) R. Antagonism of the V(1a) R by WAY 267,464 could underlie some of the prosocial effects of this drug either through a direct action or through disinhibition of oxytocin circuitry that is subject to vasopressin inhibitory influences. | | | 22420322
 |
DLK1 is a somato-dendritic protein expressed in hypothalamic arginine-vasopressin and oxytocin neurons.
Villanueva, C; Jacquier, S; de Roux, N
PloS one
7
e36134
2011
Mostrar resumen
Delta-Like 1 Homolog, Dlk1, is a paternally imprinted gene encoding a transmembrane protein involved in the differentiation of several cell types. After birth, Dlk1 expression decreases substantially in all tissues except endocrine glands. Dlk1 deletion in mice results in pre-natal and post-natal growth deficiency, mild obesity, facial abnormalities, and abnormal skeletal development, suggesting involvement of Dlk1 in perinatal survival, normal growth and homeostasis of fat deposition. A neuroendocrine function has also been suggested for DLK1 but never characterised. To evaluate the neuroendocrine function of DLK1, we first characterised Dlk1 expression in mouse hypothalamus and then studied post-natal variations of the hypothalamic expression. Western Blot analysis of adult mouse hypothalamus protein extracts showed that Dlk1 was expressed almost exclusively as a soluble protein produced by cleavage of the extracellular domain. Immunohistochemistry showed neuronal DLK1 expression in the suprachiasmatic (SCN), supraoptic (SON), paraventricular (PVN), arcuate (ARC), dorsomedial (DMN) and lateral hypothalamic (LH) nuclei. DLK1 was expressed in the dendrites and perikarya of arginine-vasopressin neurons in PVN, SCN and SON and in oxytocin neurons in PVN and SON. These findings suggest a role for DLK1 in the post-natal development of hypothalamic functions, most notably those regulated by the arginine-vasopressin and oxytocin systems. | | | 22563444
 |
Effects of RFamide-Related Peptide (RFRP)-1 and RFRP-3 on Oxytocin Release and Anxiety-Related Behaviour in Rats.
Kaewwongse M, Takayanagi Y, Onaka T.
Journal of neuroendocrinology
23
20-7
2010
Mostrar resumen
RFamide-related peptides (RFRP-1 and RFRP-3) are localised in neurones of the dorsomedial hypothalamus in rats. The dorsomedial hypothalamus plays an essential role in neuroendocrine and behavioural stress responses. In the present study, we examined the role of RFRP in the control of neuroendocrine and behavioural responses in rats. Stressful stimuli increased expression of Fos protein in RFRP-immunoreactive neurones of the dorsomedial hypothalamus, suggesting that stressful stimuli activate RFRP neurones. Intracerebroventricular injection of RFRPs increased the expression of Fos protein in oxytocin neurones in the hypothalamus and plasma concentrations of adrenocorticotrophic hormone and oxytocin. The hypothalamic paraventricular and supraoptic nuclei expressed mRNA of GPR147, the putative RFRP receptor, and application of RFRPs to isolated supraoptic nuclei facilitated oxytocin release, suggesting that RFRPs activate oxytocin neurones directly. Furthermore, the administration of RFRPs induced anxiety-related behaviour in rats in open-field tests. All these data taken together suggest that RFRPs play a role in the control of neuroendocrine and behavioural stress responses in rats. | | | 21029217
 |
Activation of different neuronal phenotypes in the rat brain induced by liver ischemia–reperfusion injury: dual Fos/neuropeptide immunohistochemistry.
J Bundzikova,Z Pirnik,L Lackovicova,B Mravec,A Kiss
Cellular and molecular neurobiology
31
2010
Mostrar resumen
The aim of the present study was to reveal the effect of liver ischemia–reperfusion injury (LIRI) on the activity of selected neuronal phenotypes in rat brain by applying dual Fos-oxytocin (OXY), vasopressin (AVP), tyrosine hydroxylase (TH), phenylethanolamine N-methyltransferase (PNMT), corticoliberine (CRH), and neuropeptide Y (NPY) immunohistochemistry. Two liver ischemia–reperfusion models were investigated: (i) single ligation of the hepatic artery (LIRIa) for 30 min and (ii) combined ligation of the portal triad (the common hepatic artery, portal vein, and common bile duct) (LIRIb) for 15 min. The animals were killed 90 min, 5 h, and 24 h after reperfusion. Intact and sham operated rats served as controls. As indicated by semiquantitative estimation, increases in the number of Fos-positive cells mainly occurred 90 min after both liver reperfusion injuries, including activation of AVP and OXY perikarya in the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei, and TH, NPY, and PNMT perikarya in the catecholaminergic ventrolateral medullar A1/C1 area. Moreover, only PNMT perikarya located in the A1/C1 cell group exhibited increased Fos expression 5 h after LIRIb reperfusion. No or very low Fos expression was found 24 h after reperfusion in neuronal phenotypes studied. Our results show that both models of the LIRI activate, almost by the same effectiveness, a number of different neuronal phenotypes which stimulation may be associated with a complex of physiological responses induced by (1) surgery (NPY, TH, PNMT), (2) hemodynamic changes (AVP, OXY, TH, PNMT), (3) inflammation evoked by ischemia and subsequent reperfusion (TH), and (4) glucoprivation induced by fasting (NPY, PNMT, TH). All these events may contribute by different strength to the development of pathological alterations occurring during the liver ischemia–reperfusion injury. | | | 21061149
 |
Oxytocin immunoreactivity in the corpus cavernosum of patients with erectile dysfunction.
Fatih Tarhan,Gökhan Faydacı,Aylin Ege Gül,Uğur Kuyumcuoğlu,Bilal Eryıldırım
Urologia internationalis
87
2010
Mostrar resumen
Oxytocin is released by the posterior pituitary gland during male orgasm. Additionally, the presence of an oxytocin receptor gene and protein expression in human corpus cavernosum is demonstrated, and it has contractile activity on the smooth muscle of the animal and human corpus cavernosum in vitro. The aim of this study was to investigate the immunoreactivity of oxytocin in corpus cavernosum of patients with organic erectile dysfunction and to compare it with healthy controls. | | | 21832819
 |
MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635.
Glenn E Hunt,Iain S McGregor,Jennifer L Cornish,Paul D Callaghan
Brain research bulletin
86
2010
Mostrar resumen
The popular party drug MDMA (3,4-methylenedioxymethamphetamine, Ecstasy) increases sociability in both humans and laboratory animals. Recent research suggests that these prosocial effects may involve serotonin (5-HT)-stimulated hypothalamic release of the neuropeptide oxytocin. WAY 100635, a 5-HT(1A) receptor antagonist, prevents MDMA-induced increases in plasma oxytocin and also reduces MDMA-mediated increases in social interaction in rats. The present study used c-Fos immunohistochemistry to determine the possible role of 5-HT(1A) receptors in MDMA-mediated activation of oxytocin synthesizing neurons. Male Wistar rats (n=8/group) were administered MDMA (10 mg/kg, i.p.) with or without WAY 100635 (1 mg/kg, i.p.) pre-treatment and c-Fos expression was then assessed throughout the brain. MDMA significantly increased locomotor activity and this effect was partly prevented by WAY 100635, in agreement with previous studies. WAY 100635 significantly reduced MDMA-induced c-Fos expression in a subset of brain regions examined. A particularly prominent reduction was seen in the oxytocin-positive neurons of the supraoptic nucleus and paraventricular hypothalamus, with more modest reductions in the Islands of Calleja, median preoptic nucleus, somatosensory cortex and nucleus of the solitary tract. WAY 100635 did not alter MDMA-induced c-Fos expression in the striatum, thalamus, or central amygdala. These results indicate that MDMA's action on oxytocin producing cells in the hypothalamus is mediated through 5-HT(1A) receptors and that certain specific cortical, limbic and brainstem sites are also activated by MDMA via these receptors. | | | 21745546
 |
Early life stress disrupts peripubertal development of aggression in male mice.
Tsuda MC, Yamaguchi N, Ogawa S
Neuroreport
22
259-63.
2010
Mostrar resumen
To investigate the effects of early life stress on the development of social behaviors in male mice, we examined behavioral responses toward same sex stimulus mice in the social investigation test and aggressive behaviors in peripubertal male mice exposed to maternal separation (MS) during the first 2 weeks of life. MS suppressed aggressive behaviors from 5-9 weeks of age, but had no effect on social investigative behaviors in the social investigation test. Investigation of neuroendocrine bases of behavioral effects of MS showed that MS reduced plasma testosterone levels and decreased arginine vasopressin and increased oxytocin immunoreactivity in the paraventricular nucleus of peripubertal males. These results collectively suggest that early life stress disrupts the development of male aggressive behaviors and associated neuroendocrine systems. | | | 21403582
 |
Noradrenergic nuclei that receive sensory input during mating and project to the ventromedial hypothalamus play a role in mating-induced pseudopregnancy in the female rat.
Northrop, LE; Polston, EK; Erskine, MS
Journal of neuroendocrinology
22
1061-71
2009
Mostrar resumen
In female rats, vaginal-cervical stimulation (VCS) received during mating induces bicircadian prolactin surges that are required for the maintenance of pregnancy or pseudopregnancy (PSP). The neural circuits that transmit VCS inputs to the brain have not been fully described, although mating stimulation is known to activate medullary noradrenergic cell groups that project to the forebrain. In response to VCS, these neurones release noradrenaline within the ventrolateral division of the ventromedial hypothalamus (VMHvl) and the posterodorsal medial amygdala (MePD), two forebrain sites that are implicated in the initiation of PSP. Noradrenaline receptor activation within the VMHvl is both necessary and sufficient for PSP induction, suggesting that noradrenaline acting within the VMHvl is particularly important in mediating the effects of VCS towards the establishment of PSP. We therefore investigated whether or not endogenous, VCS-induced noradrenaline release within the VMHvl is involved in PSP induction in the rat. Before the receipt of sufficient mating stimulation to induce PSP, a retrograde neurotoxin, dopamine-β-hydroxylase-saporin (DBH-SAP), was infused bilaterally into the either the VMHvl or the MePD to selectively destroy afferent noradrenergic nuclei in the brainstem. DBH-SAP infusions into the VMHvl lesioned mating-responsive noradrenergic neurones in A1 and A2 medullary nuclei and reduced the incidence of PSP by 50%. Infusions of DBH-SAP into the MePD had no effect on the subsequent induction of PSP. These results suggest that VCS is conveyed to mating-responsive forebrain areas by brainstem noradrenergic neurones, and that the activity of noradrenergic cells projecting to the VMHvl is involved in the induction of PSP. Artículo Texto completo | | | 20673300
 |