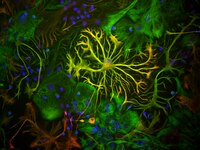
Rationally Designed MicroRNA-Based Genetic Classifiers Target Specific Neurons in the Brain.
Sayeg, MK; Weinberg, BH; Cha, SS; Goodloe, M; Wong, WW; Han, X
ACS synthetic biology
4
788-95
2015
Mostrar resumen
Targeting transgene expression to specific cell types in vivo has proven instrumental in characterizing the functional role of defined cell populations. Genetic classifiers, synthetic transgene constructs designed to restrict expression to particular classes of cells, commonly rely on transcriptional promoters to define cellular specificity. However, the large size of many natural promoters complicates their use in viral vectors, an important mode of transgene delivery in the brain and in human gene therapy. Here, we expanded upon an emerging classifier platform, orthogonal to promoter-based strategies, that exploits endogenous microRNA regulation to target gene expression. Such classifiers have been extensively explored in other tissues; however, their use in the nervous system has thus far been limited to targeting gene expression between neurons and supporting cells. Here, we tested the possibility of using combinatory microRNA regulation to specify gene targeting between neuronal subtypes, and successfully targeted inhibitory cells in the neocortex. These classifiers demonstrate the feasibility of designing a new generation of microRNA-based neuron-type- and brain-region-specific gene expression targeting neurotechnologies. | | | 25848814
 |
Organotypic vibrosections from whole brain adult Alzheimer mice (overexpressing amyloid-precursor-protein with the Swedish-Dutch-Iowa mutations) as a model to study clearance of beta-amyloid plaques.
Humpel, C
Frontiers in aging neuroscience
7
47
2015
Mostrar resumen
Alzheimer's disease is a severe neurodegenerative disorder of the brain, pathologically characterized by extracellular beta-amyloid plaques, intraneuronal Tau inclusions, inflammation, reactive glial cells, vascular pathology and neuronal cell death. The degradation and clearance of beta-amyloid plaques is an interesting therapeutic approach, and the proteases neprilysin (NEP), insulysin and matrix metalloproteinases (MMP) are of particular interest. The aim of this project was to establish and characterize a simple in vitro model to study the degrading effects of these proteases. Organoytpic brain vibrosections (120 μm thick) were sectioned from adult (9 month old) wildtype and transgenic mice (expressing amyloid precursor protein (APP) harboring the Swedish K670N/M671L, Dutch E693Q, and Iowa D694N mutations; APP_SDI) and cultured for 2 weeks. Plaques were stained by immunohistochemistry for beta-amyloid and Thioflavin S. Our data show that plaques were evident in 2 week old cultures from 9 month old transgenic mice. These plaques were surrounded by reactive GFAP+ astroglia and Iba1+ microglia. Incubation of fresh slices for 2 weeks with 1-0.1-0.01 μg/ml of NEP, insulysin, MMP-2, or MMP-9 showed that NEP, insulysin, and MMP-9 markedly degraded beta-amyloid plaques but only at the highest concentration. Our data provide for the first time a potent and powerful living brain vibrosection model containing a high number of plaques, which allows to rapidly and simply study the degradation and clearance of beta-amyloid plaques in vitro. | | | 25914642
 |
Cortical iron regulation and inflammatory response in Alzheimer's disease and APPSWE/PS1ΔE9 mice: a histological perspective.
Meadowcroft, MD; Connor, JR; Yang, QX
Frontiers in neuroscience
9
255
2015
Mostrar resumen
Disruption of iron homeostasis and increased glial response are known to occur in brains afflicted by Alzheimer's disease (AD). While the APP/PS1 transgenic mouse model recapitulates the hallmark amyloid-beta plaque pathology of AD, it does so in a different neuronal mileu than humans. Understanding the iron characteristics and glial response of the APP/PS1 model is important when testing new treatment procedures and translating these results. Brain tissue from AD patients, APP/PS1 mice, and controls were stained for iron, H- and L-ferritin, microglia, astrocytes, Aβ40∕42, and degenerating neurons. The histological data demonstrate differences in ferritin, iron distribution, gliosis, and Aβ plaque composition between APP/PS1 and AD tissue. Specifically, an association between focal iron deposition and Aβ plaques is found ubiquitously throughout the AD tissue and is not observed in the APP/PS1 mouse model. Ferritin, microglia, and astrocyte staining show differential response patterns to amyloid plaques in AD and the APP/PS1 tissue. Aβ 40 and 42 antibody and thioflavin staining demonstrate morphological differences in plaque composition. The histological data support the hypothesis that iron distribution, iron management, and glial response histologically differ between the APP/PS1 and AD brain. Acknowledging the caveat that there are distinct plaque, iron, and glial contrasts between the AD brain and the APP/PS1 mouse is crucial when utilizing this model. | | | 26257600
 |
Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer's [corrected] disease model.
Wu, Z; Guo, Z; Gearing, M; Chen, G
Nature communications
5
4159
2014
Mostrar resumen
Amyloid plaques and tau tangles are common pathological hallmarks for Alzheimer's disease (AD); however, reducing Aβ production failed to relieve the symptoms of AD patients. Here we report a high GABA (γ-aminobutyric acid) content in reactive astrocytes in the dentate gyrus (DG) of a mouse model for AD (5xFAD) that results in increased tonic inhibition and memory deficit. We also confirm in human AD patient brains that dentate astrocytes have a high GABA content, suggesting that high astrocytic GABA level may be a novel biomarker and a potential diagnostic tool for AD. The excessive GABA in 5xFAD astrocytes is released through an astrocyte-specific GABA transporter GAT3/4, and significantly enhances tonic GABA inhibition in dentate granule cells. Importantly, reducing tonic inhibition in 5xFAD mice rescues the impairment of long-term potentiation (LTP) and memory deficit. Thus, reducing tonic GABA inhibition in the DG may lead to a novel therapy for AD. | Immunohistochemistry | | 24923909
 |
Short- and long-term treatment of mouse cortical primary astrocytes with β-amyloid differentially regulates the mRNA expression of L-type calcium channels.
Daschil, N; Geisler, S; Obermair, GJ; Humpel, C
Pharmacology
93
24-31
2014
Mostrar resumen
It is well established that reactive astrocytes express L-type calcium channels (LTCC), but their functional role is completely unknown. We have recently shown that reactive astrocytes highly express the CaV1.2 α1-subunit around β-amyloid (Aβ) plaques in an Alzheimer mouse model. The aim of the present study was to explore whether Aβ peptides may regulate the mRNA expression of all LTCC subunits in primary mouse astrocytes in culture.Confluent primary astrocytes were incubated with 10 µg/ml of human or murine Aβ or the toxic fragment Aβ25-35 for 3 days or for 3 weeks. The LTCC subunits were determined by quantitative RT-PCR.Our data show that murine Aβ42 slightly but significantly increased CaV1.2 and CaV1.3 expression when incubated for 3 days. This acute treatment with murine Aβ enhanced β2 and β3 mRNA levels but decreased α2δ-2 mRNA expression. When astrocytes were incubated for 3 weeks, the levels of CaV1.2 α1 were significantly decreased by the murine Aβ and the toxic fragment. As a control, the protein kinase C-ε activator DCP-LA displayed a decrease in CaV2.1 expression.In conclusion, our data show that Aβ can differentially regulate LTCC expression in primary mouse astrocytes depending on incubation time. | | | 24435206
 |
Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice.
Niesman, IR; Schilling, JM; Shapiro, LA; Kellerhals, SE; Bonds, JA; Kleschevnikov, AM; Cui, W; Voong, A; Krajewski, S; Ali, SS; Roth, DM; Patel, HH; Patel, PM; Head, BP
Journal of neuroinflammation
11
39
2014
Mostrar resumen
Traumatic brain injury (TBI) enhances pro-inflammatory responses, neuronal loss and long-term behavioral deficits. Caveolins (Cavs) are regulators of neuronal and glial survival signaling. Previously we showed that astrocyte and microglial activation is increased in Cav-1 knock-out (KO) mice and that Cav-1 and Cav-3 modulate microglial morphology. We hypothesized that Cavs may regulate cytokine production after TBI.Controlled cortical impact (CCI) model of TBI (3 m/second; 1.0 mm depth; parietal cortex) was performed on wild-type (WT; C57Bl/6), Cav-1 KO, and Cav-3 KO mice. Histology and immunofluorescence microscopy (lesion volume, glia activation), behavioral tests (open field, balance beam, wire grip, T-maze), electrophysiology, electron paramagnetic resonance, membrane fractionation, and multiplex assays were performed. Data were analyzed by unpaired t tests or analysis of variance (ANOVA) with post-hoc Bonferroni's multiple comparison.CCI increased cortical and hippocampal injury and decreased expression of MLR-localized synaptic proteins (24 hours), enhanced NADPH oxidase (Nox) activity (24 hours and 1 week), enhanced polysynaptic responses (1 week), and caused hippocampal-dependent learning deficits (3 months). CCI increased brain lesion volume in both Cav-3 and Cav-1 KO mice after 24 hours (P less than 0.0001, n = 4; one-way ANOVA). Multiplex array revealed a significant increase in expression of IL-1β, IL-9, IL-10, KC (keratinocyte chemoattractant), and monocyte chemoattractant protein 1 (MCP-1) in ipsilateral hemisphere and IL-9, IL-10, IL-17, and macrophage inflammatory protein 1 alpha (MIP-1α) in contralateral hemisphere of WT mice after 4 hours. CCI increased IL-2, IL-6, KC and MCP-1 in ipsilateral and IL-6, IL-9, IL-17 and KC in contralateral hemispheres in Cav-1 KO and increased all 10 cytokines/chemokines in both hemispheres except for IL-17 (ipsilateral) and MIP-1α (contralateral) in Cav-3 KO (versus WT CCI). Cav-3 KO CCI showed increased IL-1β, IL-9, KC, MCP-1, MIP-1α, and granulocyte-macrophage colony-stimulating factor in ipsilateral and IL-1β, IL-2, IL-9, IL-10, and IL-17 in contralateral hemispheres (P = 0.0005, n = 6; two-way ANOVA) compared to Cav-1 KO CCI.CCI caused astrocyte and microglial activation and hippocampal neuronal injury. Cav-1 and Cav-3 KO exhibited enhanced lesion volume and cytokine/chemokine production after CCI. These findings suggest that Cav isoforms may regulate neuroinflammatory responses and neuroprotection following TBI. | | | 24593993
 |
Activity-dependent FUS dysregulation disrupts synaptic homeostasis.
Sephton, CF; Tang, AA; Kulkarni, A; West, J; Brooks, M; Stubblefield, JJ; Liu, Y; Zhang, MQ; Green, CB; Huber, KM; Huang, EJ; Herz, J; Yu, G
Proceedings of the National Academy of Sciences of the United States of America
111
E4769-78
2014
Mostrar resumen
The RNA-binding protein fused-in-sarcoma (FUS) has been associated with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD), two neurodegenerative disorders that share similar clinical and pathological features. Both missense mutations and overexpression of wild-type FUS protein can be pathogenic in human patients. To study the molecular and cellular basis by which FUS mutations and overexpression cause disease, we generated novel transgenic mice globally expressing low levels of human wild-type protein (FUS(WT)) and a pathological mutation (FUS(R521G)). FUS(WT) and FUS(R521G) mice that develop severe motor deficits also show neuroinflammation, denervated neuromuscular junctions, and premature death, phenocopying the human diseases. A portion of FUS(R521G) mice escape early lethality; these escapers have modest motor impairments and altered sociability, which correspond with a reduction of dendritic arbors and mature spines. Remarkably, only FUS(R521G) mice show dendritic defects; FUS(WT) mice do not. Activation of metabotropic glutamate receptors 1/5 in neocortical slices and isolated synaptoneurosomes increases endogenous mouse FUS and FUS(WT) protein levels but decreases the FUS(R521G) protein, providing a potential biochemical basis for the dendritic spine differences between FUS(WT) and FUS(R521G) mice. | | | 25324524
 |
Vascular pathology of 20-month-old hypercholesterolemia mice in comparison to triple-transgenic and APPSwDI Alzheimer's disease mouse models.
Hohsfield, LA; Daschil, N; Orädd, G; Strömberg, I; Humpel, C
Molecular and cellular neurosciences
63
83-95
2014
Mostrar resumen
Several studies have shown that elevated plasma cholesterol levels (i.e. hypercholesterolemia) serve as a risk factor for late-onset Alzheimer's disease (AD). However, it remains unclear how hypercholesterolemia may contribute to the onset and progression of AD pathology. In order to determine the role of hypercholesterolemia at various stages of AD, we evaluated the effects of high cholesterol diet (5% cholesterol) in wild-type (WT; C57BL6) and triple-transgenic AD (3xTg-AD; Psen1, APPSwe, tauB301L) mice at 7, 14, and 20 months. The transgenic APP-Swedish/Dutch/Iowa AD mouse model (APPSwDI) was used as a control since these animals are more pathologically-accelerated and are known to exhibit extensive plaque deposition and cerebral amyloid angiopathy. Here, we describe the effects of high cholesterol diet on: (1) cognitive function and stress, (2) AD-associated pathologies, (3) neuroinflammation, (4) blood–brain barrier disruption and ventricle size, and (5) vascular dysfunction. Our data show that high dietary cholesterol increases weight, slightly impairs cognitive function, promotes glial cell activation and complement-related pathways, enhances the infiltration of blood-derived proteins and alters vascular integrity, however, it does not induce AD-related pathologies. While normal-fed 3xTg-AD mice display a typical AD-like pathology in addition to severe cognitive impairment and neuroinflammation at 20 months of age, vascular alterations are less pronounced. No microbleedings were seen by MRI, however, the ventricle size was enlarged. Triple-transgenic AD mice, on the other hand, fed a high cholesterol diet do not survive past 14 months of age. Our data indicates that cholesterol does not markedly potentiate AD-related pathology, nor does it cause significant impairments in cognition. However, it appears that high cholesterol diet markedly increases stress-related plasma corticosterone levels as well as some vessel pathologies. Together, our findings represent the first demonstration of prolonged high cholesterol diet and the examination of its effects at various stages of cerebrovascular- and AD-related disease. | | | 25447943
 |
Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer's disease.
Kim, HY; Kim, HV; Yoon, JH; Kang, BR; Cho, SM; Lee, S; Kim, JY; Kim, JW; Cho, Y; Woo, J; Kim, Y
Scientific reports
4
7467
2014
Mostrar resumen
Alzheimer's disease (AD) is a lethal progressive neurological disorder affecting the memory. Recently, US Food and Drug Administration mitigated the standard for drug approval, allowing symptomatic drugs that only improve cognitive deficits to be allowed to accelerate on to clinical trials. Our study focuses on taurine, an endogenous amino acid found in high concentrations in humans. It has demonstrated neuroprotective properties against many forms of dementia. In this study, we assessed cognitively enhancing property of taurine in transgenic mouse model of AD. We orally administered taurine via drinking water to adult APP/PS1 transgenic mouse model for 6 weeks. Taurine treatment rescued cognitive deficits in APP/PS1 mice up to the age-matching wild-type mice in Y-maze and passive avoidance tests without modifying the behaviours of cognitively normal mice. In the cortex of APP/PS1 mice, taurine slightly decreased insoluble fraction of Aβ. While the exact mechanism of taurine in AD has not yet been ascertained, our results suggest that taurine can aid cognitive impairment and may inhibit Aβ-related damages. | | | 25502280
 |
Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice.
Ataka, K; Asakawa, A; Nagaishi, K; Kaimoto, K; Sawada, A; Hayakawa, Y; Tatezawa, R; Inui, A; Fujimiya, M
PloS one
8
e81744
2013
Mostrar resumen
Microglia of the central nervous system act as sentinels and rapidly react to infection or inflammation. The pathophysiological role of bone marrow-derived microglia is of particular interest because they affect neurodegenerative disorders and neuropathic pain. The hypothesis of the current study is that chronic psychological stress (chronic PS) induces the infiltration of bone marrow-derived microglia into hypothalamus by means of chemokine axes in brain and bone marrow.Here we show that bone marrow-derived microglia specifically infiltrate the paraventricular nucleus (PVN) of mice that received chronic PS. Bone marrow derived-microglia are CX3CR1(low)CCR2(+)CXCR4(high), as distinct from CX3CR1(high)CCR2(-)CXCR4(low) resident microglia, and express higher levels of interleukin-1β (IL-1β) but lower levels of tumor necrosis factor-α (TNF-α). Chronic PS stimulates the expression of monocyte chemotactic protein-1 (MCP-1) in PVN neurons, reduces stromal cell-derived factor-1 (SDF-1) in the bone marrow and increases the frequency of CXCR4(+) monocytes in peripheral circulation. And then a chemokine (C-C motif) receptor 2 (CCR2) or a β3-adrenoceptor blockade prevents infiltration of bone marrow-derived microglia in the PVN.Chronic PS induces the infiltration of bone marrow-derived microglia into PVN, and it is conceivable that the MCP-1/CCR2 axis in PVN and the SDF-1/CXCR4 axis in bone marrow are involved in this mechanism. | | | 24303068
 |