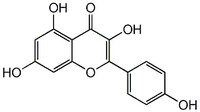
420345 Sigma-AldrichKaempferol - CAS 520-18-3 - Calbiochem
A cell-permeable phytoestrogen that inhibits topoisomerase I-catalyzed DNA religation in HL-60 cells.
More>> A cell-permeable phytoestrogen that inhibits topoisomerase I-catalyzed DNA religation in HL-60 cells. Less<<Synonyms: 3,4ʹ,5,7-Tetrahydroxyflavone
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 520-18-3 | C₁₅H₁₀O₆ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 420345-25MG |
|
Glass bottle | 25 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 520-18-3 |
| ATP Competitive | N |
| Form | Yellow solid |
| Hill Formula | C₁₅H₁₀O₆ |
| Chemical formula | C₁₅H₁₀O₆ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Topoisomerase I-catalyzed DNA religation in HL-60 cells |
| Primary Target IC<sub>50</sub> | 180 µM against COX-1 activity |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | LK9275200 |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 420345-25MG | 04055977187502 |
Documentation
Kaempferol - CAS 520-18-3 - Calbiochem SDS
| Title |
|---|
Kaempferol - CAS 520-18-3 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 420345 |
References
| Reference overview |
|---|
| Wang, C.N., et al. 2001. J. Biol. Chem. 276, 5287. Sloley, B.D., et al. 2000. J. Pharm. Pharmacol. 52, 451. Wang, H., et al. 2000. Phytomedicine 7, 15. Liang, Y.C., et al. 1999. Carcinogenesis 20, 1945. Roth, A., et al. 1999. J. Neurosci. Res. 57, 399. Boege, F., et al. 1996. J. Biol. Chem. 271, 2262. Constantinou, A., et al. 1995. J. Nat. Prod. 58, 217. |
Brochure
| Title |
|---|
| Alzheimer's Disease Brochure & Technical Guide |
| Caspases and other Apoptosis Related Tools Brochure |