Estrogen and telomerase in human peripheral blood mononuclear cells.
Ann L Benko,Nancy J Olsen,William J Kovacs
Molecular and cellular endocrinology
364
2011
Mostra il sommario
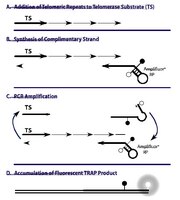
The enzyme telomerase plays an important role in sustaining the capacity of T lymphocytes for homeostatic replication. Recent data have suggested that gonadal steroids might modulate telomerase expression or activity within these cells. We used quantitative assay techniques for both telomerase mRNA expression and telomerase enzymatic activity to systematically examine the effects of physiologic concentrations of estradiol on human peripheral blood mononuclear cells under basal conditions and under conditions that normally enhance telomerase activity in T lymphocytes. Cells from women tended to exhibit higher responsiveness of telomerase activity to induction by T cell receptor engagement. However, we found no evidence of a direct effect of physiologic concentrations of estradiol on human telomerase reverse transcriptase (hTERT) mRNA expression, hTERT protein expression, or telomerase enzymatic activity in cultured PBMCs. While estrogen might exert developmental effects on T cells to alter telomerase responsiveness to T cell receptor engagement, mature peripheral T cells do not respond to estradiol with changes in expression or function of telomerase. | 22954679
 |
Abeta peptides accelerate the senescence of endothelial cells in vitro and in vivo, impairing angiogenesis.
Sandra Donnini,Raffaella Solito,Elisa Cetti,Federico Corti,Antonio Giachetti,Silvia Carra,Monica Beltrame,Franco Cotelli,Marina Ziche
FASEB journal : official publication of the Federation of American Societies for Experimental Biology
24
2009
Mostra il sommario
Cerebral amyloid angiopathy (CAA) caused by amyloid beta (Abeta) deposition around brain microvessels results in vascular degenerative changes. Antiangiogenic Abeta properties are known to contribute to the compromised cerebrovascular architecture. Here we hypothesize that Abeta peptides impair angiogenesis by causing endothelial cells to enter senescence at an early stage of vascular development. Wild-type (WT) Abeta and its mutated variant E22Q peptide, endowed with marked vascular tropism, were used in this study. In vivo, in zebrafish embryos, the WT or E22Q peptides reduced embryo survival with an IC(50) of 6.1 and 4.7 microM, respectively. The 2.5 microM concentration, showing minimal toxicity, was chosen. Alkaline phosphatase staining revealed disorganized vessel patterning, narrowing, and reduced branching of vessels. Beta-galactosidase staining and the cyclin-dependent kinase inhibitor p21 expression, indicative of senescence, were increased. In vitro, WT and E22Q reduced endothelial cell survival with an IC(50) of 12.3 and 8.8 microM, respectively. The 5 microM concentration, devoid of acute effects on the endothelium, was applied chronically to long-term cultured human umbilical vein endothelial cells (HUVECs). We observed reduced cumulative population doubling, which coincided with beta-galactosidase accumulation, down-regulation of telomerase reverse-transcriptase mRNA expression, decreased telomerase activity, and p21 activation. Senescent HUVECs showed marked angiogenesis impairment, as Abeta treatment reduced tube sprouting. The endothelial injuries caused by the E22Q peptide were much more aggressive than those induced by the WT peptide. Premature Abeta-induced senescence of the endothelium, producing progressive alterations of microvessel morphology and functions, may represent one of the underlying mechanisms for sporadic or heritable CAA. | 20207941
 |
Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration.
Simó S, Jossin Y, Cooper JA
J Neurosci
30
5668-76.
2009
Mostra il sommario
The multilayered mammalian neocortex develops by the coordinated immigration and differentiation of cells that are produced at distant sites. Correct layering requires an extracellular protein, Reelin (Reln), an intracellular signaling molecule, Disabled-1 (Dab1), and an E3 ubiquitin ligase, Cullin-5 (Cul5). Reln activates Dab1, which is then degraded by Cul5. Here we test whether Cul5 regulates neuron layering by affecting Dab1 stability or other mechanisms. We find that a stabilized mutant Dab1, which resists Cul5-dependent degradation, causes a similar phenotype to Cul5 deficiency. Moreover, Cul5 has no effect when Dab1 is absent. The effects of Cul5 and Dab1 are cell autonomous, and Cul5 regulates movement of early as well as late cortical neurons. Removing Cul5 increases the speed at which neurons migrate through the cortical plate by reducing the time spent stationary and increasing the speed of individual steps. These results show that Cul5 regulates neuron layering by stimulating Dab1 degradation and that Cul5 controls migration speed and stopping point, and they demonstrate the importance of negative feedback in signaling during cortical development. | 20410119
 |