534032 Sigma-AldrichNOX Inhibitor IV, GKT136901 - CAS 955272-06-7 - Calbiochem
Potent, orally available dual inhibitor of NOX1/NOX4. Blocks NOX-mediated ROS outburst and efficiently scavenges peroxynitrite.
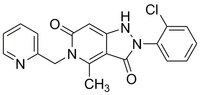
More>> Potent, orally available dual inhibitor of NOX1/NOX4. Blocks NOX-mediated ROS outburst and efficiently scavenges peroxynitrite. Less<<Sinonimi: 2-(2-Chlorophenyl)-4-methyl-5-(pyridin-2-ylmethyl)-1H-pyrazolo-[4,3-c]pyridine-3,6(2H,5H)-dione, NADPH Oxidase Inhibitor IV, Dual NOX4/NOX1 Inhibitor, GKT-136901
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Tabella delle specifiche principali
| CAS # | Empirical Formula |
|---|---|
| 955272-06-7 | C₁₉H₁₅ClN₄O₂ |
Prezzi e disponibilità
| Numero di catalogo | Disponibilità | Confezionamento | Qtà/conf | Prezzo | Quantità | |
|---|---|---|---|---|---|---|
| 5340320001 |
|
Bottiglia di vetro | 10 mg |
|
— |
| References | |
|---|---|
| References | Schildknecht, S., et al. 2014. Curr. Med. Chem. 21, 365. Laleu, B., et al. 2010. J. Med. Chem. 53, 7715. Sedeek, M., et al. 2010. Am. J. Physiol. Renal Physiol. 299, F1348. |
| Product Information | |
|---|---|
| CAS number | 955272-06-7 |
| Form | Pale yellow to brown solid |
| Hill Formula | C₁₉H₁₅ClN₄O₂ |
| Chemical formula | C₁₉H₁₅ClN₄O₂ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | NOX1- and NOX4-containing NADPH oxidase activity |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 5340320001 | 04055977281767 |
Documentation
NOX Inhibitor IV, GKT136901 - CAS 955272-06-7 - Calbiochem MSDS
| Titolo |
|---|
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Schildknecht, S., et al. 2014. Curr. Med. Chem. 21, 365. Laleu, B., et al. 2010. J. Med. Chem. 53, 7715. Sedeek, M., et al. 2010. Am. J. Physiol. Renal Physiol. 299, F1348. |