420350 Sigma-AldrichICMT Inhibitor - Calbiochem
The ICMT Inhibitor controls the biological activity of ICMT. This small molecule/inhibitor is primarily used for Cancer applications.
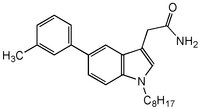
More>> The ICMT Inhibitor controls the biological activity of ICMT. This small molecule/inhibitor is primarily used for Cancer applications. Less<<Sinonimi: 2-(5-(3-Methylphenyl)-1-octyl-1H-indol-3-yl)acetamide, 2-(1-Octyl-5-m-tolyl-1H-indol-3-yl) acetamide, Cysmethynil, Cysmethynil, Isoprenylcysteine Carboxyl Methyltransferase Inhibitor
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Prezzi e disponibilità
| Numero di catalogo | Disponibilità | Confezionamento | Qtà/conf | Prezzo | Quantità | |
|---|---|---|---|---|---|---|
| 420350-10MG |
|
Bottiglia di vetro | 10 mg |
|
— |
| Product Information | |
|---|---|
| Form | Pale yellow semi-solid |
| Hill Formula | C₂₅H₃₂N₂0 |
| Chemical formula | C₂₅H₃₂N₂0 |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 420350-10MG | 04055977187519 |
Documentation
ICMT Inhibitor - Calbiochem MSDS
| Titolo |
|---|
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Go, M.L., et al. 2010. J. Med. Chem. 53, 6838; Wang, M., et al. 2008. J. Biol. Chem. 283, 18678; Baron, R.A., et al. 2007. Biochemistry 46, 554; Winter-Vann, A.M., et al. 2005. Proc. Natl. Acad. Sci. USA 102, 4336. |