530030 Sigma-AldrichGlutaminase Inhibitor II, BPTES - CAS 314045-39-1 - Calbiochem
A cell-permeable, potent, selective, reversible, and uncompetitive allosteric inhibitor of kidney-type glutaminase (GLS1). Does not affect the activity of liver glutaminase.
More>> A cell-permeable, potent, selective, reversible, and uncompetitive allosteric inhibitor of kidney-type glutaminase (GLS1). Does not affect the activity of liver glutaminase. Less<<Sinonimi: N,Nʹ-(5,5ʹ-(2,2ʹ-Thiobis(ethane-2,1-diyl))bis(1,3,4-thiadiazole-5,2-diyl))bis(2-phenylacetamide), Kidney-Type Glutaminase Inhibitor II, GAC Inhibitor II, GLS1 Inhibitor II, KGA Inhibitor II
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Tabella delle specifiche principali
| CAS # | Empirical Formula |
|---|---|
| 314045-39-1 | C₂₄H₂₄N₆O₂S₃ |
Prezzi e disponibilità
| Numero di catalogo | Disponibilità | Confezionamento | Qtà/conf | Prezzo | Quantità | |
|---|---|---|---|---|---|---|
| 5300300001 |
|
Bottiglia di vetro | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 314045-39-1 |
| Form | Yellow powder |
| Hill Formula | C₂₄H₂₄N₆O₂S₃ |
| Chemical formula | C₂₄H₂₄N₆O₂S₃ |
| Reversible | Y |
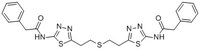
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | kidney-type glutaminase |
| Primary Target IC<sub>50</sub> | 140 and 210 nM in HEK293 cells expressing wt-KGA and F318Y-cKGA, respectively |
| Purity | ≥94% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 5300300001 | 04055977241198 |
Documentation
Glutaminase Inhibitor II, BPTES - CAS 314045-39-1 - Calbiochem MSDS
| Titolo |
|---|
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Shukla, K., et al. 2012. J. Med. Chem. 55, 10551. Thangavelu, K., et al. 2012. Proc. Natl. Acad. Sci. USA 109, 7705. Le, A., et al. 2012. Cell Metab. 15, 110. DeLaBarre, B., et al. 2011. Biochemistry 50, 10764. Seltzer, M.J., et al. 2010. Cancer Res. 70, 8981. Robinson, M.M., et al. 2007. Biochem. J. 406, 407. |