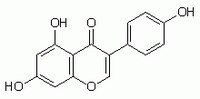
345834 Sigma-AldrichGenistein, Soybean - CAS 446-72-0 - Calbiochem
A cell-permeable, reversible, substrate competitive inhibitor of protein tyrosine kinases, including autophosphorylation of epidermal growth factor receptor kinase (IC₅₀ = 2.6 µM).
More>> A cell-permeable, reversible, substrate competitive inhibitor of protein tyrosine kinases, including autophosphorylation of epidermal growth factor receptor kinase (IC₅₀ = 2.6 µM). Less<<Sinonimi: 4ʹ,5,7-Trihydroxyisoflavone
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Tabella delle specifiche principali
| CAS # | Empirical Formula |
|---|---|
| 446-72-0 | C₁₅H₁₀O₅ |
Prezzi e disponibilità
| Numero di catalogo | Disponibilità | Confezionamento | Qtà/conf | Prezzo | Quantità | |
|---|---|---|---|---|---|---|
| 345834-20MG |
|
Bottiglia di vetro | 20 mg |
|
— | |
| 345834-50MG |
|
Fiala di plastica | 50 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 446-72-0 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₅H₁₀O₅ |
| Chemical formula | C₁₅H₁₀O₅ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | EGFR kinase |
| Primary Target IC<sub>50</sub> | 2.6 µM against autophosphorylation of epidermal growth factor receptor kinase |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | NR2392000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 345834-20MG | 04055977214376 |
| 345834-50MG | 04055977214383 |
Documentation
Genistein, Soybean - CAS 446-72-0 - Calbiochem MSDS
| Titolo |
|---|
Genistein, Soybean - CAS 446-72-0 - Calbiochem Certificati d'Analisi
| Titolo | Numero di lotto |
|---|---|
| 345834 |
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Constantinou, A., and Huberman, E. 1995. Proc. Soc. Exp. Biol. Med. 208, 109. Wei, H., et al. 1995. Proc. Soc. Exp. Biol. Med. 208, 124. Wei, H., et al. 1995. Carcinogenesis 17, 73. Kobayashi, S., et al. 1994. J. Biol. Chem. 269, 9011. Migita, K., et al. 1994. J. Immunol. 153, 3457. Spinozzi, F., et al. 1994. Leuk. Res. 18, 431. Dhar, A., et al. 1990. Mol. Pharmacol. 37, 519. Hill, T.D., et al. 1990. Science 248, 1660. Dean, N.M., et al. 1989. Biochem. Biophys. Res. Commun. 165, 795. Akiyama, T., et al. 1987. J. Biol. Chem. 262, 5592. |
Brochure
| Titolo |
|---|
| Caspases and other Apoptosis Related Tools Brochure |
Citazioni
| Titolo | |
|---|---|
|
|