Differential effects of antidepressants escitalopram versus lithium on Gs alpha membrane relocalization.
Donati, RJ; Schappi, J; Czysz, AH; Jackson, A; Rasenick, MM
BMC neuroscience
16
40
2015
Mostra il sommario
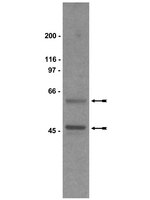
Plasma membrane localization can play a significant role in the ultimate function of certain proteins. Specific membrane domains like lipid rafts have been shown to be inhibitory domains to a number of signaling proteins, including Gsα, and chronic antidepressant treatment facilitates Gs signaling by removing Gsα form lipid rafts. The intent of this study is to compare the effects of the selective serotnin reuptake inhibitor, escitalopram, with that of the mood stabilizing drug, lithium.There are a number of mechanisms of action proposed for lithium as a mood stabilizing agent, but the interactions between G proteins (particularly Gs) and mood stabilizing drugs are not well explored. Of particular interest was the possibility that there was some effect of mood stabilizers on the association between Gsα and cholesterol-rich membrane microdomains (lipid rafts), similar to that seen with long-term antidepressant treatment. This was examined by biochemical and imaging (fluorescence recovery after photobleaching: FRAP) approaches. Results indicate that escitalopram was effective at liberating Gsα from lipid rafts while lithium was not.There are a number of drug treatments for mood disorders and yet there is no unifying hypothesis for a cellular or molecular basis of action. It is evident that there may in fact not be a single mechanism, but rather a number of different mechanisms that converge at a common point. The results of this study indicate that the mood stabilizing agent, lithium, and the selective serotonin reuptake inhibitor, escitalopram, act on their cellular targets through mutually exclusive pathways. These results also validate the hypothesis that translocation of Gsα from lipid rafts could serve as a biosignature for antidepressant action. | 26162823
 |
Parathyroid hormone signaling via Gαs is selectively inhibited by an NH(2)-terminally truncated Gαs: implications for pseudohypoparathyroidism.
Svetlana Puzhko,Cynthia Gates Goodyer,Mohammad Amin Kerachian,Lucie Canaff,Madhusmita Misra,Harald Jüppner,Murat Bastepe,Geoffrey N Hendy
Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research
26
2010
Mostra il sommario
Pseudohypoparathyroid patients have resistance predominantly to parathyroid hormone (PTH), and here we have examined the ability of an alternative Gαs-related protein to inhibit Gαs activity in a hormone-selective manner. We tested whether the GNAS exon A/B-derived NH(2)-terminally truncated (Tr) αs protein alters stimulation of adenylate cyclase by the PTH receptor (PTHR1), the thyroid-stimulating hormone (TSH) receptor (TSHR), the β(2)-adrenergic receptor (β(2)AR), or the AVP receptor (V2R). HEK293 cells cotransfected with receptor and full-length (FL) Gαs ± Tr αs protein expression vectors were stimulated with agonists (PTH [10(-7) to 10(-9) M], TSH [1 to 100 mU], isoproterenol [10(-6) to 10(-8) M], or AVP [10(-6) to 10(-8) M]). Following PTH stimulation, HEK293 cells cotransfected with PTHR1 + FL Gαs + Tr αs had a significantly lower cAMP response than those transfected with only PTHR1 + FL Gαs. Tr αs also exerted an inhibitory effect on the cAMP levels stimulated by TSH via the TSHR but had little or no effect on isoproterenol or AVP acting via β(2)AR or V2R, respectively. These differences mimic the spectrum of hormone resistance in pseudohypoparathyroidism type 1a (PHP-1a) and type 1b (PHP-1b) patients. In opossum kidney (OK) cells, endogenously expressing the PTHR1 and β(2)AR, the exogenous expression of Tr αs at a level similar to endogenous FL Gαs resulted in blunting of the cAMP response to PTH, whereas that to isoproterenol was unaltered. A pseudopseudohypoparathyroid patient with Albright hereditary osteodystrophy harbored a de novo paternally inherited M1I Gαs mutation. Similar maternally inherited mutations at the initiation codon have been identified previously in PHP-1a patients. The M1I αs mutant (lacking the first 59 amino acids of Gαs) blunted the increase in cAMP levels stimulated via the PTHR1 in both HEK293 and OK cells similar to the Tr αs protein. Thus NH(2)-terminally truncated forms of Gαs may contribute to the pathogenesis of pseudohypoparathyroidism by inhibiting the activity of Gαs itself in a GPCR selective manner. | 21713996
 |
Transmembrane adenylyl cyclase regulates amphibian sperm motility through protein kinase A activation.
O'Brien, ED; Krapf, D; Cabada, MO; Visconti, PE; Arranz, SE
Developmental biology
350
80-8
2010
Mostra il sommario
Sperm motility is essential for achieving fertilization. In animals with external fertilization as amphibians, spermatozoa are stored in a quiescent state in the testis. Spermiation to hypotonic fertilization media triggers activation of sperm motility. Bufo arenarum sperm are immotile in artificial seminal plasma (ASP) but acquire in situ flagellar beating upon dilution. In addition to the effect of low osmolarity on sperm motility activation, we report that diffusible factors of the egg jelly coat (EW) regulate motility patterns, switching from in situ to progressive movement. The signal transduction pathway involved in amphibian sperm motility activation is mostly unknown. In the present study, we show a correlation between motility activation triggered by low osmotic pressure and activation of protein kinase A (PKA). Moreover, this is the first study to present strong evidences that point toward a role of a transmembrane adenyl-cyclase (tmAC) in the regulation of amphibian sperm motility through PKA activation. | 21126515
 |
Arrestin-3 is essential for the activation of Fyn by the luteinizing hormone receptor (LHR) in MA-10 cells.
Colette Galet,Mario Ascoli
Cellular signalling
20
2008
Mostra il sommario
Recent studies showed that Fyn is a mediator of the LHR-induced activation of the ERK1/2 cascade in MA-10 cells. Since the LHR is a G protein-coupled receptor and the Src family of kinases can be activated by some Galpha subunits and by the non-visual arrestins we investigated the role of these signaling molecules in the LHR-provoked activation of Fyn. Small interfering RNAs (siRNAs) that target two Galpha subunits that participate in LHR signaling (Galpha(s) and Galpha(11)) and one that targets arrestin-3 were co-transfected with the hLHR in MA-10 cells. We then determined the effects of these siRNAs on the LHR-provoked activation of Fyn, the phosphorylation of FAK (a prominent Fyn substrate) and the release of EGF-like growth factors (a Fyn-mediated process). Expression of the siRNA against Galpha(s) decreased the level of Galpha(s) and LHR-stimulated cAMP production by approximately 50% but did not affect LHR-stimulated Fyn activation or FAK phosphorylation. Likewise, expression of the siRNA against Galpha(11) decreased the level of Galpha(11) and LHR-stimulated inositol phosphate production by approximately 50% but did not affect LHR-stimulated Fyn activation or FAK phosphorylation. Expression of the siRNA against arrestin-3 decreased the level of arrestin-3 and the rate of internalization of hCG by approximately 50% and it also inhibited the LHR-provoked stimulation of Fyn, the phosphorylation of FAK and the release of EGF-like growth factors. These results show that, in MA-10 cells, the hLHR activates Fyn through an arrestin-3-dependent pathway and that this pathway is a mediator of the hLHR-provoked release of EGF-like growth factors. Testo completo dell'articolo | 18647647
 |