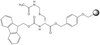
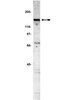
Farnesyltransferase inhibitor FTI-277 reduces mortality of septic mice along with improved bacterial clearance.
Yang, W; Yamada, M; Tamura, Y; Chang, K; Mao, J; Zou, L; Feng, Y; Kida, K; Scherrer-Crosbie, M; Chao, W; Ichinose, F; Yu, YM; Fischman, AJ; Tompkins, RG; Yao, S; Kaneki, M
The Journal of pharmacology and experimental therapeutics
339
832-41
2010
Mostra il sommario
Treatment with statins, inhibitors of HMG-CoA reductase, extends the survival of septic mice. However, the molecular mechanisms underlying the cholesterol-lowering, independent beneficial effects of statins in sepsis are poorly understood. The inhibition of protein isoprenylation, namely farnesylation and geranylgeranylation, has been proposed as a mediator of the pleiotropic protective effects of statins, although direct evidence is lacking. Major features of sepsis-induced immune suppression include T-cell dysfunction, which is characterized by apoptosis of splenic T cells, increased CD4(+)Foxp3(+) regulatory T cells (Tregs), and suppression of type 1 helper T-cell response [e.g., interferon-γ (IFN-γ) secretion] in mice. Here, we show that the induction of sepsis by cecal ligation and puncture (CLP) resulted in increases in farnesyltransferase activity and farnesylated proteins in the spleen relative to sham operation. Treatment with farnesyltransferase inhibitor N-[4-[2(R)-amino-3-mercaptopropyl]amino-2-phenylbenzoyl]methionine methyl ester trifluoroacetate salt (FTI-277) (25 mg/kg b.wt. i.p.) at 2 h after CLP blocked the increase in farnesylated proteins and improved survival and bacterial clearance of septic mice. FTI-277 reverted to or mitigated sepsis-induced apoptosis in spleen and thymus, increased splenic CD4(+)Foxp3(+) Tregs, and suppressed IFN-γ secretion and proliferation of splenocytes in response to anti-CD3+CD28 antibodies in mice. Moreover, FTI-277 promoted macrophage phagocytotic activity in septic mice. These results indicate that elevation in protein farnesylation plays a role in derangements in immune function and mortality of septic mice. These findings suggest that prevention of immune dysfunction might contribute to FTI-277-induced improvement in survival of septic mice. These data highlight protein farnesyltransferase as a novel potential molecular target to reduce the mortality of patients with sepsis. | 21873557
 |
RhoB prenylation is driven by the three carboxyl-terminal amino acids of the protein: evidenced in vivo by an anti-farnesyl cysteine antibody.
Baron, R, et al.
Proc. Natl. Acad. Sci. U.S.A., 97: 11626-31 (2000)
1999
Mostra il sommario
Protein isoprenylation is a lipid posttranslational modification required for the function of many proteins that share a carboxyl-terminal CAAX motif. The X residue determines which isoprenoid will be added to the cysteine. When X is a methionine or serine, the farnesyl-transferase transfers a farnesyl, and when X is a leucine or isoleucine, the geranygeranyl-transferase I, a geranylgeranyl group. But despite its CKVL motif, RhoB was reported to be both geranylgeranylated and farnesylated. Thus, the determinants of RhoB prenylation appear more complex than initially thought. To determine the role of RhoB CAAX motif, we designed RhoB mutants with modified CAAX sequence expressed in baculovirus-infected insect cells. We demonstrated that RhoB was prenylated as a function of the three terminal amino acids, i.e., RhoB bearing the CAIM motif of lamin B or CLLL motif of Rap1A was farnesylated or geranylgeranylated, respectively. Next, we produced a specific polyclonal antibody against farnesyl cysteine methyl ester allowing prenylation analysis avoiding the metabolic labeling restrictions. We confirmed that the unique modification of the RhoB CAAX box was sufficient to direct the RhoB distinct prenylation in mammalian cells and, inversely, that a RhoA-CKVL chimera could be alternatively prenylated. Moreover, the immunoprecipitation of endogenous RhoB from cells with the anti-farnesyl cysteine antibody suggested that wild-type RhoB is farnesylated in vivo. Taken together, our results demonstrated that the three last carboxyl amino acids are the main determinants for RhoB prenylation and described an anti-farnesyl cysteine antibody as a useful tool for understanding the cellular control of protein farnesylation. | 11027361
 |
Isoprenylation of polypeptides in the nematode Caenorhabditis elegans.
Aspbury, R A, et al.
Biochim. Biophys. Acta, 1392: 265-75 (1998)
1998
Mostra il sommario
Covalent modification of eucaryotic proteins, involving addition of isoprenyl groups, is a widespread phenomenon. Here we provide direct evidence for this form of covalent modification in the free-living nematode, Caenorhabditis elegans. Following incubation in the presence of [3H]mevalonolactone, specific C. elegans polypeptides became labelled in both aqueous and detergent (Triton X-114)-enriched extracts. Chemical and GC-MS analysis of modifying groups, cleaved from C. elegans polypeptides, revealed that geranylgeranylation and, to a lesser extent, farnesylation of target polypeptides occurred. Immunoblot analysis provided preliminary evidence that the ras-like let-60 polypeptide was a target for isoprenylation in C. elegans. | 9630668
 |