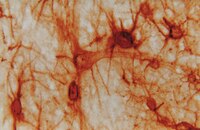
Enriched housing enhances recovery of limb placement ability and reduces aggrecan-containing perineuronal nets in the rat somatosensory cortex after experimental stroke.
Madinier, A; Quattromani, MJ; Sjölund, C; Ruscher, K; Wieloch, T
PloS one
9
e93121
2014
Mostra il sommario
Stroke causes life long disabilities where few therapeutic options are available. Using electrical and magnetic stimulation of the brain and physical rehabilitation, recovery of brain function can be enhanced even late after stroke. Animal models support this notion, and housing rodents in an enriched environment (EE) several days after experimental stroke stimulates lost brain function by multisensory mechanisms. We studied the dynamics of functional recovery of rats with a lesion to the fore and hind limb motor areas induced by photothrombosis (PT), and with subsequent housing in either standard (STD) or EE. In this model, skilled motor function is not significantly enhanced by enriched housing, while the speed of recovery of sensori-motor function substantially improves over the 9-week study period. In particular, this stroke lesion completely obliterates the fore and hind limb placing ability when visual and whisker guidance is prevented, a deficit that persists for up to 9 weeks of recovery, but that is markedly restored within 2 weeks by enriched housing. Enriched housing after stroke also leads to a significant loss of perineuronal net (PNN) immunoreactivity; detection of aggrecan protein backbone with AB1031 antibody was decreased by 13-22%, and labelling of a glycan moiety of aggrecan with Cat-315 antibody was reduced by 25-30% in the peri-infarct area and in the somatosensory cortex, respectively. The majority of these cells are parvalbumin/GABA inhibitory interneurons that are important in sensori-information processing. We conclude that damage to the fore and hind limb motor areas provides a model of loss of limb placing response without visual guidance, a deficit also seen in more than 50% of stroke patients. This loss is amenable to recovery induced by multiple sensory stimulation and correlates with a decrease in aggrecan-containing PNNs around inhibitory interneurons. Modulating the PNN structure after ischemic damage may provide new therapies enhancing tactile/proprioceptive function after stroke. | Immunohistochemistry | Rat | 24664200
 |
Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer's disease.
Lendvai, Dávid, et al.
Acta Neuropathol., 125: 215-29 (2013)
2013
Mostra il sommario
Perineuronal matrix is an extracellular protein scaffold to shape neuronal responsiveness and survival. Whilst perineuronal nets engulf the somatodendritic axis of neurons, axonal coats are focal extracellular protein aggregates surrounding individual synapses. Here, we addressed the chemical identity and subcellular localization of both perineuronal and perisynaptic matrices in the human hippocampus, whose neuronal circuitry is progressively compromised in Alzheimer's disease. We hypothesized that (1) the cellular expression sites of chondroitin sulphate proteoglycan-containing extracellular matrix associate with specific neuronal identities, reflecting network dynamics, and (2) the regional distribution and molecular composition of axonal coats must withstand Alzheimer's disease-related modifications to protect functional synapses. We show by epitope-specific antibodies that the perineuronal protomap of the human hippocampus is distinct from other mammals since pyramidal cells but not calretinin(+) and calbindin(+) interneurons, neurochemically classified as novel neuronal subtypes, lack perineuronal nets. We find that cartilage link protein-1 and brevican-containing matrices form isolated perisynaptic coats, engulfing both inhibitory and excitatory terminals in the dentate gyrus and entorhinal cortex. Ultrastructural analysis revealed that presynaptic neurons contribute components of perisynaptic coats via axonal transport. We demonstrate, by combining biochemical profiling and neuroanatomy in Alzheimer's patients and transgenic (APdE9) mice, the preserved turnover and distribution of axonal coats around functional synapses along dendrite segments containing hyperphosphorylated tau and in amyloid-β-laden hippocampal microdomains. We conclude that the presynapse-driven formation of axonal coats is a candidate mechanism to maintain synapse integrity under neurodegenerative conditions. | | | 22961619
 |
Disturbance of perineuronal nets in the perilesional area after photothrombosis is not associated with neuronal death.
Magdalena Karetko-Sysa,Jolanta Skangiel-Kramska,Dorota Nowicka
Experimental neurology
231
2010
Mostra il sommario
Perineuronal nets (PNNs) are a condensed form of extracellular matrix that covers the surface of a subset of neurons. Their presence limits neuronal plasticity and may protect neurons against harmful agents. Here we analyzed the relationship between spatiotemporal changes in PNN expression and cell death markers after focal cortical photothrombotic stroke in rats. We registered a substantial decrease in PNN density using Wisteria floribunda agglutinin staining and CAT-315 and brevican immunoreactivity; the decrease occurred not only in the lesion core but also in the perilesional and remote cortex as well as in homotopic contralateral cortical regions. Fluoro Jade C and TUNEL staining in perilesional and remote areas, however, showed a low density of dying cells. Our results suggest that the PNN reduction was not a result of cellular death and could be considered an attempt to create conditions favorable for synaptic remodeling. | | | 21683696
 |
Distribution of perineuronal nets in the human superior olivary complex.
Schmidt E, Wolski TP Jr, Kulesza RJ Jr
Hear Res
2009
Mostra il sommario
Perineuronal nets (PNNs) are specialized assemblies of chondroitin sulfate proteoglycans (CSPGs) in the central nervous system that form a lattice-like covering over the cell body, primary dendrites and initial axon segment of select neuronal populations. PNNs appear to play significant roles in development of the central nervous system, neuronal protection, synaptic plasticity and local ion homeostasis. In seven human brainstems (average age=81 years), we have utilized Wisteria floribunda (WFA) histochemistry and immunocytochemistry for CSPG to map the distribution of PNNs within the nuclei of the human superior olivary complex (SOC). Within the SOC, the majority of net-bearing neurons are situated in the most medially situated nuclei, especially the superior paraolivary nucleus and medial nucleus of the trapezoid body. Net-bearing neurons are consistently found in the ventral nucleus of the trapezoid body and posterior periolivary nucleus, but to a lesser extent in the lateral nucleus of the trapezoid body. Finally, perineuronal nets are typically absent from the lateral and medial superior olives. Copyright © 2010 Elsevier B.V. All rights reserved. | | | 20307636
 |
A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex.
Lander, C, et al.
J. Neurosci., 17: 1928-39 (1997)
1997
Mostra il sommario
Monoclonal antibody Cat-301 recognizes a chondroitin sulfate proteoglycan (CSPG) expressed on the extracellular surface of cell bodies and proximal dendrites of specific subsets of neurons in many areas of the mammalian CNS, including the cat visual cortex. The Cat-301 CSPG is first detected at the close of the critical period in development, a period during which the pattern of neuronal activity determines the mature synaptic circuitry and neuronal phenotype. In the cat visual cortex, dark-rearing from birth prolongs the duration of the critical period and attenuates the expression of the Cat-301 antigen, implicating the Cat-301 CSPG in the cellular mechanisms that terminate the period of synaptic plasticity. Because the Cat-301 antigen is expressed on only a limited subset of neurons, we have further examined the molecular heterogeneity among neuronal cell-surface CSPGs and have asked (1) whether other neuronal subsets carry distinct CSPGs and (2) whether the activity-dependent expression of the Cat-301 CSPG is a property generalizable to related cell-surface CSPGs. Here, we report two new monoclonal antibodies, Cat-315 and Cat-316, which together with Cat-301 define a family of at least seven related yet distinct CSPGs. These three antibodies define nonidentical subsets of neurons in the cat visual cortex. The expression of normal levels of these CSPGs is reduced by dark-rearing. Together, these data show that the family of cell-surface CSPGs is molecularly diverse, that different sets of neurons express distinct complements of cell-surface antigens, and that the regulation of CSPG expression by activity may be a general feature of neuronal cell-surface CSPGs. | | | 9045722
 |