119136 Sigma-AldrichAdenosine A1 Receptor Agonist II, CCPA - CAS 37739-05-2 - Calbiochem
Sinonimi: (2R,3R,4S,5R)-2-(2-chloro-6-(cyclopentylamino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol, 2-Chloro-N6-cyclopentyladenosine
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Prezzi e disponibilità
| Numero di catalogo | Disponibilità | Confezionamento | Qtà/conf | Prezzo | Quantità | |
|---|---|---|---|---|---|---|
| 119136-10MG |
|
Bottiglia di vetro | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 37739-05-2 |
| Form | White solid |
| Hill Formula | C₁₅H₂₀ClN₅O₄ |
| Chemical formula | C₁₅H₂₀ClN₅O₄ |
| Reversible | Y |
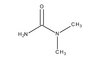
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | A1 |
| Primary Target K<sub>i</sub> | 2.3 nM |
| Purity | ≥99% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 119136-10MG | 04055977223323 |
Documentation
Adenosine A1 Receptor Agonist II, CCPA - CAS 37739-05-2 - Calbiochem MSDS
| Titolo |
|---|
Adenosine A1 Receptor Agonist II, CCPA - CAS 37739-05-2 - Calbiochem Certificati d'Analisi
| Titolo | Numero di lotto |
|---|---|
| 119136 |
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Burgdorf, C. et al. 2005. J Cardiovasc Pharmacol. 45, 1. Klotz, K. N., et al. 2000. Naunyn Schmiedebergs Arch Pharmacol. 362, 382. Mironov, S., et al. 1990. J Neurophysiol. 81, 1. Monopoli, A., et al. 1994.Arzneimittelforschung. 44, 1305. Concas, A., et al. 1993. J Pharmacol Exp Ther. 267, 844. Lohse, M. J., et al. 1988. Nauryn Schmiedebergs Arch Pharmacol. 337, 687. Coffin, V. L., et al. 1987. J Pharmacol Exp Ther. 241, 76. |