420220 Sigma-AldrichiPSC Induction Enhancer, Thiazovivin - CAS 1226056-71-8 - Calbiochem
iPSC Induction Enhancer, Thiazovivin, CAS 1226056-71-8, is a cell-permeable ROCK inhibitor that prevents trypsinization-induced apoptosis in hESC cultures.
More>> iPSC Induction Enhancer, Thiazovivin, CAS 1226056-71-8, is a cell-permeable ROCK inhibitor that prevents trypsinization-induced apoptosis in hESC cultures. Less<<Synonymes: N-Benzyl-2-(pyrimidin-4-ylamino)thiazole-4-carboxamide, Induced Pluripotent Stem Cells Induction Enhancer, Rho Kinase Inhibitor XII
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
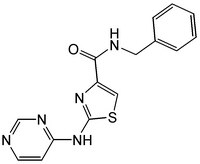
| 1226056-71-8 | C₁₅H₁₃N₅OS |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 420220-10MG |
|
Ampoule plast. | 10 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable aminothiazolo-carboxamide compound that is ~5-fold more potent than Y-27632 (Cat. Nos. 688000 & 688001) as a ROCK inhibitor (71% inhibition at 2 µM). Effectively prevents trypsinization-induced apoptosis in hESC cultures by stabilizing newly synthesized cell surface E-cadherin and cell-cell adhesion. Unlike, Ptn (Cat. No. 544049), Tzv protects dissociated hESCs even in the absence of ECM. Greatly augments SB431542 (Cat. No. 616461) and PD0325901 (Cat. No. 444966) in improving iPSCs generation efficiency from 4-TF- (Oct44, Klf4, Sox2, and c-Myc) transfected fibroblast cultures. |
| Catalogue Number | 420220 |
| Brand Family | Calbiochem® |
| Synonyms | N-Benzyl-2-(pyrimidin-4-ylamino)thiazole-4-carboxamide, Induced Pluripotent Stem Cells Induction Enhancer, Rho Kinase Inhibitor XII |
| References | |
|---|---|
| References | Xu, Y., et al. 2010. Proc. Natl. Acad. Sci. USA 107, 8129 Lin, T., et al. 2009. Nat. Methods 6, 805. |
| Product Information | |
|---|---|
| CAS number | 1226056-71-8 |
| Form | Tan powder |
| Hill Formula | C₁₅H₁₃N₅OS |
| Chemical formula | C₁₅H₁₃N₅OS |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | iPSC Induction Enhancer, Thiazovivin, CAS 1226056-71-8, is a cell-permeable ROCK inhibitor that prevents trypsinization-induced apoptosis in hESC cultures. |
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 420220-10MG | 04055977210811 |
Documentation
iPSC Induction Enhancer, Thiazovivin - CAS 1226056-71-8 - Calbiochem FDS
| Titre |
|---|
iPSC Induction Enhancer, Thiazovivin - CAS 1226056-71-8 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 420220 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Xu, Y., et al. 2010. Proc. Natl. Acad. Sci. USA 107, 8129 Lin, T., et al. 2009. Nat. Methods 6, 805. |
| Fiche technique | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|