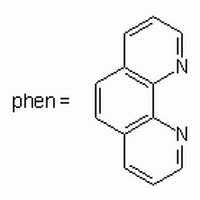
203695 Sigma-AldrichbpV(phen) - CAS 42494-73-5 - Calbiochem
bpV(phen, CAS 42494-73-5, is an insulin mimetic and an inhibitor of protein phosphotyrosine phosphatase. More potent in dephosphorylating of autophosphorylated insulin receptor than orthovanadate.
More>> bpV(phen, CAS 42494-73-5, is an insulin mimetic and an inhibitor of protein phosphotyrosine phosphatase. More potent in dephosphorylating of autophosphorylated insulin receptor than orthovanadate. Less<<Synonymes: Potassium Bisperoxo(1,10-phenanthroline)oxovanadate (V), PTEN Inhibitor I, PTP Inhibitor VIII
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 42494-73-5 | K[VO(O₂)₂C₁₂H₈N₂] |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 203695-10MG |
|
Ampoule plast. | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 42494-73-5 |
| ATP Competitive | N |
| Form | Yellow solid |
| Hill Formula | K[VO(O₂)₂C₁₂H₈N₂] |
| Chemical formula | K[VO(O₂)₂C₁₂H₈N₂] |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | PTEN |
| Primary Target IC<sub>50</sub> | 38 nM |
| Purity | ≥99% by ⁵¹V-NMR |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 203695-10MG | 07790788048365 |
Documentation
bpV(phen) - CAS 42494-73-5 - Calbiochem FDS
| Titre |
|---|
bpV(phen) - CAS 42494-73-5 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 203695 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Schmid, A.C., et al. 2004. FEBS Lett. 566, 35. Muzyamba, M.C., et al. 1999. J. Physiol. 517, 421. Drake, P.G., et al. 1996. Endocrinology 137, 4960. Bevan, A.P., et al. 1995. Am. J. Physiol. 268, E60. Bevan, A.P., et al. 1995. J. Biol. Chem. 270, 10784. Yale, J.F., et al. 1995. Diabetes 44, 1274. Posner, B.I., et al. 1994. J. Biol. Chem. 269, 4596. |
Brochure
| Titre |
|---|
| Protein Phosphatases Technical Bulletin |