682160 Sigma-AldrichXestospongin C, Xestospongia sp. - Calbiochem
Xestospongin C, Xestospongia sp. CAS 88903-69-9, is a highly potent, reversible, and membrane-permeable blocker of IP3-mediated Ca2+ release (IC50 = 358 nM).
More>> Xestospongin C, Xestospongia sp. CAS 88903-69-9, is a highly potent, reversible, and membrane-permeable blocker of IP3-mediated Ca2+ release (IC50 = 358 nM). Less<<Synonymes: XeC
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| Empirical Formula |
|---|
| C₂₈H₅₀N₂O₂ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 682160-10UG |
|
Ampoule plast. | 10 μg |
|
— |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | White to off-white film |
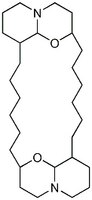
| Hill Formula | C₂₈H₅₀N₂O₂ |
| Chemical formula | C₂₈H₅₀N₂O₂ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | Xestospongin C, Xestospongia sp. CAS 88903-69-9, is a highly potent, reversible, and membrane-permeable blocker of IP3-mediated Ca2+ release (IC50 = 358 nM). |
| Biological Information | |
|---|---|
| Primary Target | IP3-mediated Ca2+ release |
| Primary Target IC<sub>50</sub> | 358 nM in blocking IP3-mediated Ca2+ release |
| Purity | ≥98% by TLC or HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 682160-10UG | 07790788052355 |
Documentation
Xestospongin C, Xestospongia sp. - Calbiochem FDS
| Titre |
|---|
Xestospongin C, Xestospongia sp. - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 682160 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Oka, T., et al. 2002. Br. J. Pharmacol. 135, 1959. Gafni, J., et al. 1997. Neuron 19, 723. Tinker, A., and Williams, A.J. 1995. Biophys. J. 68, 111. Callaway, C., et al. 1994. J. Biol. Chem. 269, 15876. Quirion, J.C., et al. 1992. J. Nat. Prod. 55, 1505. Nakagawa, M., et al. 1984. Tetrahedron Lett. 25, 3227. |
Citations
| Titre | |
|---|---|
|
|
| Fiche technique | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|