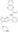567860 Sigma-AldrichSRT1720 - CAS 925434-55-5 - Calbiochem
SRT1720, CAS 925434-55-5, is a cell-permeable inhibitor of the mitochondrial SIRT3. Inhibition is AceCS2-competitive (Ki = 0.56 µM; Km = 2.44 µM), but NAD+-uncompetitive (Ki = 0.34 µM; Km = 280 µM).
More>> SRT1720, CAS 925434-55-5, is a cell-permeable inhibitor of the mitochondrial SIRT3. Inhibition is AceCS2-competitive (Ki = 0.56 µM; Km = 2.44 µM), but NAD+-uncompetitive (Ki = 0.34 µM; Km = 280 µM). Less<<Synonymes: N-(2-(3-(1-Piperazinylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)-2-quinolinecarboxamide, Sirtuin-3 Inhibitor, SRT1720, HAT Inhibitor XI, Histone Acetyltransferase Inhibitor XI, p300/CBP Inhibitor IX, SIRT3 Inhibitor II
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 925434-55-5 | C₂₅H₂₃N₇OS |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 567860-10MG |
|
Flacon en verre | 10 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable quinolinecarboxamide compound that is shown to inhibit the mitochondrial SIRT3 in a substrate AceCS2-competitive (Ki = 0.56 µM; Km = 2.44 µM), but NAD+-uncompetitive (Ki = 0.34 µM; Km = 280 µM), manner. Also reported to decrease cellular p53 Lys382 acetylation (Effective conc. = 10 µM in U2OS and MEF cultures) and inhibit p300 HAT activity (IC50 = 9 µM) in vitro, as well as offer therapeutic benefits in several murine and rodent type 2 diabetes models (100 mg/kg/dayl p.o.) in vivo. Whether and how SRT1720 activates SIRT1 activity remains uncertain. Also available as a 25 mM solution in DMSO (Cat. No. 530748). |
| Catalogue Number | 567860 |
| Brand Family | Calbiochem® |
| Synonyms | N-(2-(3-(1-Piperazinylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)-2-quinolinecarboxamide, Sirtuin-3 Inhibitor, SRT1720, HAT Inhibitor XI, Histone Acetyltransferase Inhibitor XI, p300/CBP Inhibitor IX, SIRT3 Inhibitor II |
| Product Information | |
|---|---|
| CAS number | 925434-55-5 |
| Form | Yellow solid |
| Hill Formula | C₂₅H₂₃N₇OS |
| Chemical formula | C₂₅H₂₃N₇OS |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 567860-10MG | 04055977190380 |
Documentation
SRT1720 - CAS 925434-55-5 - Calbiochem FDS
| Titre |
|---|
SRT1720 - CAS 925434-55-5 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 567860 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Baur, J.A., et al. 2012. Nat. Rev. Drug Discov. 11, 443. Minor, R.K., et al. 2011. Sci. Rep. 1, 70. Huber, J.L., et al. 2010. Future Med. Chem. 2, 1751 Pacholec, M., et al. 2010. J. Biol. Chem. 285, 8340 Jin, L., et al. 2009. Protein Sci. 18, 514 Feige, J.N., et al. 2008. Cell Metab. 8, 347 Milne, J.C., et al. 2007. Nature 450, 712. |