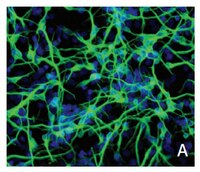
A dynamic view of the proteomic landscape during differentiation of ReNcell VM cells, an immortalized human neural progenitor line.
Song, Y; Subramanian, K; Berberich, MJ; Rodriguez, S; Latorre, IJ; Luria, CM; Everley, R; Albers, MW; Mitchison, TJ; Sorger, PK
Sci Data
6
190016
2019
Afficher le résumé
The immortalized human ReNcell VM cell line represents a reproducible and easy-to-propagate cell culture system for studying the differentiation of neural progenitors. To better characterize the starting line and its subsequent differentiation, we assessed protein and phospho-protein levels and cell morphology over a 15-day period during which ReNcell progenitors differentiated into neurons, astrocytes and oligodendrocytes. Five of the resulting datasets measured protein levels or states of phosphorylation based on tandem-mass-tag (TMT) mass spectrometry and four datasets characterized cellular phenotypes using high-content microscopy. Proteomic analysis revealed reproducible changes in pathways responsible for cytoskeletal rearrangement, cell phase transitions, neuronal migration, glial differentiation, neurotrophic signalling and extracellular matrix regulation. Proteomic and imaging data revealed accelerated differentiation in cells treated with the poly-selective CDK and GSK3 inhibitor kenpaullone or the HMG-CoA reductase inhibitor mevastatin, both of which have previously been reported to promote neural differentiation. These data provide in-depth information on the ReNcell progenitor state and on neural differentiation in the presence and absence of drugs, setting the stage for functional studies. | 30778261
 |
A three-dimensional human neural cell culture model of Alzheimer's disease.
Choi, SH; Kim, YH; Hebisch, M; Sliwinski, C; Lee, S; D'Avanzo, C; Chen, H; Hooli, B; Asselin, C; Muffat, J; Klee, JB; Zhang, C; Wainger, BJ; Peitz, M; Kovacs, DM; Woolf, CJ; Wagner, SL; Tanzi, RE; Kim, DY
Nature
2014
Afficher le résumé
Alzheimer's disease is the most common form of dementia, characterized by two pathological hallmarks: amyloid-β plaques and neurofibrillary tangles. The amyloid hypothesis of Alzheimer's disease posits that the excessive accumulation of amyloid-β peptide leads to neurofibrillary tangles composed of aggregated hyperphosphorylated tau. However, to date, no single disease model has serially linked these two pathological events using human neuronal cells. Mouse models with familial Alzheimer's disease (FAD) mutations exhibit amyloid-β-induced synaptic and memory deficits but they do not fully recapitulate other key pathological events of Alzheimer's disease, including distinct neurofibrillary tangle pathology. Human neurons derived from Alzheimer's disease patients have shown elevated levels of toxic amyloid-β species and phosphorylated tau but did not demonstrate amyloid-β plaques or neurofibrillary tangles. Here we report that FAD mutations in β-amyloid precursor protein and presenilin 1 are able to induce robust extracellular deposition of amyloid-β, including amyloid-β plaques, in a human neural stem-cell-derived three-dimensional (3D) culture system. More importantly, the 3D-differentiated neuronal cells expressing FAD mutations exhibited high levels of detergent-resistant, silver-positive aggregates of phosphorylated tau in the soma and neurites, as well as filamentous tau, as detected by immunoelectron microscopy. Inhibition of amyloid-β generation with β- or γ-secretase inhibitors not only decreased amyloid-β pathology, but also attenuated tauopathy. We also found that glycogen synthase kinase 3 (GSK3) regulated amyloid-β-mediated tau phosphorylation. We have successfully recapitulated amyloid-β and tau pathology in a single 3D human neural cell culture system. Our unique strategy for recapitulating Alzheimer's disease pathology in a 3D neural cell culture model should also serve to facilitate the development of more precise human neural cell models of other neurodegenerative disorders. | 25307057
 |
Transcriptional signature and memory retention of human-induced pluripotent stem cells.
Marchetto, Maria C N, et al.
PLoS ONE, 4: e7076 (2009)
2009
Afficher le résumé
Genetic reprogramming of somatic cells to a pluripotent state (induced pluripotent stem cells or iPSCs) by over-expression of specific genes has been accomplished using mouse and human cells. However, it is still unclear how similar human iPSCs are to human Embryonic Stem Cells (hESCs). Here, we describe the transcriptional profile of human iPSCs generated without viral vectors or genomic insertions, revealing that these cells are in general similar to hESCs but with significant differences. For the generation of human iPSCs without viral vectors or genomic insertions, pluripotent factors Oct4 and Nanog were cloned in episomal vectors and transfected into human fetal neural progenitor cells. The transient expression of these two factors, or from Oct4 alone, resulted in efficient generation of human iPSCs. The reprogramming strategy described here revealed a potential transcriptional signature for human iPSCs yet retaining the gene expression of donor cells in human reprogrammed cells free of viral and transgene interference. Moreover, the episomal reprogramming strategy represents a safe way to generate human iPSCs for clinical purposes and basic research. | 19763270
 |