Deficiency of NPGPx, an oxidative stress sensor, leads to obesity in mice and human.
Chang, YC; Yu, YH; Shew, JY; Lee, WJ; Hwang, JJ; Chen, YH; Chen, YR; Wei, PC; Chuang, LM; Lee, WH
EMBO molecular medicine
5
1165-79
2013
Afficher le résumé
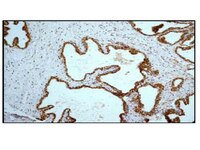
Elevated oxidative stress is closely associated with obesity. Emerging evidence shows that instead of being a consequence of obesity, oxidative stress may also contribute to fat formation. Nonselenocysteine-containing phospholipid hydroperoxide glutathione peroxidase (NPGPx) is a conserved oxidative stress sensor/transducer and deficiency of NPGPx causes accumulation of reactive oxygen species (ROS). In this communication, we show that NPGPx was highly expressed in preadipocytes of adipose tissue. Deficiency of NPGPx promoted preadipocytes to differentiate to adipocytes via ROS-dependent dimerization of protein kinase A regulatory subunits and activation of CCAAT/enhancer-binding protein beta (C/EBPβ). This enhanced adipogenesis was alleviated by antioxidant N-acetylcysteine (NAC). Consistently, NPGPx-deficient mice exhibited markedly increased fat mass and adipocyte hypertrophy, while treatment with NAC ablated these phenotypes. Furthermore, single nucleotide polymorphisms (SNPs) in human NPGPx gene, which correlated with lower NPGPx expression level in adipose tissue, were associated with higher body mass index (BMI) in several independent human populations. These results indicate that NPGPx protects against fat accumulation in mice and human via modulating ROS, and highlight the importance of targeting redox homeostasis in obesity management. | Western Blotting | 23828861
 |
Differential regulation of MeCP2 phosphorylation in the CNS by dopamine and serotonin.
Hutchinson, AN; Deng, JV; Aryal, DK; Wetsel, WC; West, AE
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology
37
321-37
2011
Afficher le résumé
Systemic administration of amphetamine (AMPH) induces phosphorylation of MeCP2 at Ser421 (pMeCP2) in select populations of neurons in the mesolimbocortical brain regions. Because AMPH simultaneously activates multiple monoamine neurotransmitter systems, here we examined the ability of dopamine (DA), serotonin (5-HT), and norepinephrine (NE) to induce pMeCP2. Selective blockade of the DA transporter (DAT) or the 5-HT transporter (SERT), but not the NE transporter (NET), was sufficient to induce pMeCP2 in the CNS. DAT blockade induced pMeCP2 in the prelimbic cortex (PLC) and nucleus accumbens (NAc), whereas SERT blockade induced pMeCP2 only in the NAc. Administration of selective DA and 5-HT receptor agonists was also sufficient to induce pMeCP2; however, the specific combination of DA and 5-HT receptors activated determined the regional- and cell-type specificity of pMeCP2 induction. The D(1)-class DA receptor agonist SKF81297 induced pMeCP2 widely; however, coadministration of the D(2)-class agonist quinpirole restricted the induction of pMeCP2 to GABAergic interneurons of the NAc. Intra-striatal injection of the adenylate cyclase activator forskolin was sufficient to induce pMeCP2 in medium-spiny neurons, suggesting that the combinatorial regulation of cAMP by different classes of DA and 5-HT receptors may contribute to the cell-type specificity of pMeCP2 induction. Consistent with the regulation of pMeCP2 by multiple monoamine neurotransmitters, genetic disruption of any single monoamine transporter in DAT-, SERT-, and NET-knockout mice failed to eliminate AMPH-induced pMeCP2 in the NAc. Together, these studies indicate that combinatorial signaling through DA and 5-HT receptors can regulate the brain region- and cell-type specific pMeCP2 in the CNS. | Western Blotting | 21956448
 |
Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons.
Kaufman, AM; Milnerwood, AJ; Sepers, MD; Coquinco, A; She, K; Wang, L; Lee, H; Craig, AM; Cynader, M; Raymond, LA
The Journal of neuroscience : the official journal of the Society for Neuroscience
32
3992-4003
2011
Afficher le résumé
The NMDAR plays a unique and vital role in subcellular signaling. Calcium influx initiates signaling cascades important for both synaptic plasticity and survival; however, overactivation of the receptor leads to toxicity and cell death. This dichotomy is partially explained by the subcellular location of the receptor. NMDARs located at the synapse stimulate cell survival pathways, while extrasynaptic receptors signal for cell death. Thus far, this interplay between synaptic and extrasynaptic NMDARs has been studied exclusively in cortical (CTX) and hippocampal neurons. It was unknown whether other cell types, such as GABAergic medium-sized spiny projection neurons of the striatum (MSNs), which bear the brunt of neurodegeneration in Huntington's disease, follow the same pattern. Here we report synaptic versus extrasynaptic NMDAR signaling in striatal MSNs and resultant activation of cAMP response element binding protein (CREB), in rat primary corticostriatal cocultures. Similarly to CTX, we found in striatal MSNs that synaptic NMDARs activate CREB, whereas extrasynaptic NMDARs dominantly oppose CREB activation. However, MSNs are much less susceptible to NMDA-mediated toxicity than CTX cells and show differences in subcellular GluN2B distribution. Blocking NMDARs with memantine (30 μm) or GluN2B-containing receptors with ifenprodil (3 μm) prevents CREB shutoff effectively in CTX and MSNs, and also rescues both neuronal types from NMDA-mediated toxicity. This work may provide cell and NMDAR subtype-specific targets for treatment of diseases with putative NMDAR involvement, including neurodegenerative disorders and ischemia. | | 22442066
 |
The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons.
John Meitzen,Danielle D Grove,Paul G Mermelstein
Endocrinology
153
2011
Afficher le résumé
Early exposure to the steroid sex hormone testosterone and its estrogen metabolite estradiol masculinize neural tissue during a developmental critical period. Many aspects of neuron anatomy and physiology are permanently altered, including later sensitivity to estradiol. Although it is well established that early hormone exposure alters neuronal responsiveness regarding classical estradiol actions (i.e. acting via nuclear estrogen receptors), it has not yet been determined whether it also alters neuronal processing of nonclassical estrogen receptor signaling, including the actions of membrane-associated estrogen receptors. Hence, we tested whether membrane estrogen receptor regulation of cAMP response element binding protein (CREB) phosphorylation observed in female (but not male) hippocampal pyramidal neurons is due to the lack of androgen and/or estrogen exposure in females during this critical period. Female rat neonates on postnatal d 0 and 1 were systemically injected with one of four compounds: vehicle, testosterone, the nonaromatizable androgen dihydrotestosterone, or estradiol. On postnatal d 2, primary hippocampal neuron cultures were generated from these animals. After 8-9 d in culture, we assessed whether estradiol affected CREB phosphorylation. Neurons from female neonates exposed to testosterone lacked estradiol signaling to CREB. In contrast, dihydrotestosterone injections of female neonates did not disrupt estradiol regulation of CREB. Estradiol injections of female neonates, however, eliminated estradiol signaling to CREB. These findings indicate that testosterone aromatization to estradiol leads to a masculinization/defeminization process whereby hippocampal neurons fail to exhibit rapid estradiol signaling to CREB. Broadly, these findings extend the organizational and aromatization hypotheses to rapid, nonclassical hormone action. | | 22865367
 |
Characterisation of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1).
Pearce LR, Alton GR, Richter DT, Kath JC, Lingardo L, Chapman J, Hwang C, Alessi DR
Biochem J
2009
Afficher le résumé
S6K1 is activated by insulin and growth factors via the PI3K and mTOR signalling pathways. S6K1 regulates numerous processes such as protein synthesis, growth, proliferation and longevity and its inhibition has been proposed as a strategy for the treatment of cancer and insulin resistance. Here we describe a novel cell-permeable inhibitor of S6K1, PF-4708671, which specifically inhibits the S6K1 isoform with a Ki of 20 nM and IC50 of 160 nM. PF-4708671 prevents the S6K1-mediated phosphorylation of S6 protein in response to IGF1 while having no effect upon the TPA-induced phosphorylation of substrates of the highly related RSK and MSK kinases. PF-4708671 was also found to induce phosphorylation of the T-loop and hydrophobic motif of S6K1, an effect that is dependent upon mTORC1. PF-4708671 is the first S6K1 specific inhibitor to be reported and will be a useful tool for delineating S6K1-specific roles downstream of mTOR. | | 20704563
 |
Science (New York, N.Y.)
Ginty, DD; Kornhauser, JM; Thompson, MA; Bading, H; Mayo, KE; Takahashi, JS; Greenberg, ME
Science (New York, N.Y.)
260
238-41
1992
Afficher le résumé
Mammalian circadian rhythms are regulated by a pacemaker within the suprachiasmatic nuclei (SCN) of the hypothalamus. The molecular mechanisms controlling the synchronization of the circadian pacemaker are unknown; however, immediate early gene (IEG) expression in the SCN is tightly correlated with entrainment of SCN-regulated rhythms. Antibodies were isolated that recognize the activated, phosphorylated form of the transcription factor cyclic adenosine monophosphate response element binding protein (CREB). Within minutes after exposure of hamsters to light, CREB in the SCN became phosphorylated on the transcriptional regulatory site, Ser133. CREB phosphorylation was dependent on circadian time: CREB became phosphorylated only at times during the circadian cycle when light induced IEG expression and caused phase shifts of circadian rhythms. These results implicate CREB in neuronal signaling in the hypothalamus and suggest that circadian clock gating of light-regulated molecular responses in the SCN occurs upstream of phosphorylation of CREB. | | 8097062
 |