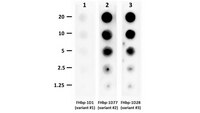
A broadly cross-reactive monoclonal antibody against an epitope on the n-terminus of meningococcal fHbp
David M Vu 1 , Rolando Pajon, Donald C Reason, Dan M Granoff
Sci Rep
2
341
2011
Afficher le résumé
Meningococcal factor H binding protein (fHbp) is an important vaccine antigen for prevention of disease caused by capsular group B strains. The protein has been sub-classified into three variant groups. Most anti-fHbp antibodies are variant group-specific and recognize epitopes on the C-terminal domain. We report a murine IgG1 mAb, JAR 41, which broadly cross-reacted with fHbp sequence variants from all variant groups. The mAb bound to the surface of live meningococci with fHbp from each of the three variant groups. In combination with second non-bactericidal anti-fHbp mAbs, JAR 41 elicited complement-mediated bactericidal activity in vitro, and augmented passive protection against meningococcal bacteremia in human fH transgenic rats. The epitope was located on a conserved region of the N-terminal portion of the fHbp molecule opposite that of fH contact residues. The data underscore the importance of broadly cross-reactive, surface-exposed epitopes on the N-terminal domain in the design of protective fHbp vaccines. | 22461972
 |
Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor h-binding protein.
Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM.
Infect Immun
76(9)
4232-40
2008
Afficher le résumé
No broadly protective vaccine is available for the prevention of group B meningococcal disease. One promising candidate is factor H-binding protein (fHbp), which is present in all strains but often sparsely expressed. We prepared seven murine immunoglobulin G monoclonal antibodies (MAbs) against fHbp from antigenic variant group 2 (v.2) or v.3 ( approximately 40% of group B strains). Although none of the MAbs individually elicited bactericidal activity with human complement, all had activity in different combinations. We used MAb reactivity with strains expressing fHbp polymorphisms and site-specific mutagenesis to identify residues that are important for epitopes recognized by six of the v.2 or v.3 MAbs and by two v.1 MAbs that were previously characterized. Residues affecting v.2 or v.3 epitopes resided between amino acids 174 and 216, which formed an eight-stranded beta-barrel in the C domain, while residues affecting the v.1 epitopes included amino acids 121 and 122 of the B domain. Pairs of MAbs were bactericidal when their respective epitopes involved residues separated by 16 to 20 A and when at least one of the MAbs inhibited the binding of fH, a downregulatory complement protein. In contrast, there was no cooperative bactericidal activity when the distance between residues was >or=27 A or <or=14 A, which correlated with the inhibition of the binding of one MAb by the other MAb. Thus, a model for anti-fH MAb bactericidal activity against strains expressing low levels of fHbp requires the binding of two MAbs directed at nonoverlapping epitopes, which activates the classical complement pathway as well as inhibits fH binding. The latter increases the susceptibility of the organism to complement-mediated bacteriolysis. | 18591239
 |