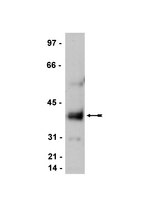
In silico screening for palmitoyl substrates reveals a role for DHHC1/3/10 (zDHHC1/3/11)-mediated neurochondrin palmitoylation in its targeting to Rab5-positive endosomes.
Oku, S; Takahashi, N; Fukata, Y; Fukata, M
The Journal of biological chemistry
288
19816-29
2013
Afficher le résumé
Protein palmitoylation, a common post-translational lipid modification, plays an important role in protein trafficking and functions. Recently developed palmitoyl-proteomic methods identified many novel substrates. However, the whole picture of palmitoyl substrates has not been clarified. Here, we performed global in silico screening using the CSS-Palm 2.0 program, free software for prediction of palmitoylation sites, and selected 17 candidates as novel palmitoyl substrates. Of the 17 candidates, 10 proteins, including 6 synaptic proteins (Syd-1, transmembrane AMPA receptor regulatory protein (TARP) γ-2, TARP γ-8, cornichon-2, Ca(2+)/calmodulin-dependent protein kinase IIα, and neurochondrin (Ncdn)/norbin), one focal adhesion protein (zyxin), two ion channels (TRPM8 and TRPC1), and one G-protein-coupled receptor (orexin 2 receptor), were palmitoylated. Using the DHHC palmitoylating enzyme library, we found that all tested substrates were palmitoylated by the Golgi-localized DHHC3/7 subfamily. Ncdn, a regulator for neurite outgrowth and synaptic plasticity, was robustly palmitoylated by the DHHC1/10 (zDHHC1/11; z1/11) subfamily, whose substrate has not yet been reported. As predicted by CSS-Palm 2.0, Cys-3 and Cys-4 are the palmitoylation sites for Ncdn. Ncdn was specifically localized in somato-dendritic regions, not in the axon of rat cultured neurons. Stimulated emission depletion microscopy revealed that Ncdn was localized to Rab5-positive early endosomes in a palmitoylation-dependent manner, where DHHC1/10 (z1/11) were also distributed. Knockdown of DHHC1, -3, or -10 (z11) resulted in the loss of Ncdn from Rab5-positive endosomes. Thus, through in silico screening, we demonstrate that Ncdn and the DHHC1/10 (z1/11) and DHHC3/7 subfamilies are novel palmitoyl substrate-enzyme pairs and that Ncdn palmitoylation plays an essential role in its specific endosomal targeting. | | 23687301
 |
Calpain-mediated regulation of stargazin in adult rat brain.
Yu, L; Rostamiani, K; Hsu, YT; Wang, Y; Bi, X; Baudry, M
Neuroscience
178
13-20
2010
Afficher le résumé
Changes in AMPA receptors have been proposed to underlie changes in synaptic efficacy in hippocampus and other brain structures. Calpain activation has also been discussed as a potential mechanism to produce lasting modifications of synaptic structure and function. Stargazin is a member of the family of transmembrane AMPA receptor associated proteins (TARPs), which participates in trafficking of AMPA receptors and regulates their kinetic properties. We report here that preincubation of thin (20 μm) frozen rat brain sections with calcium changes the immunological properties of stargazin, an effect totally blocked by a calpain inhibitor. Immunocytochemistry indicates that in situ calpain activation produces a decreased immunoreactivity for stargazin in the neuropil throughout the brain, and Western blots confirmed that a similar treatment decreased stargazin levels. Interestingly, the same treatment did not modify the immunoreactivity for another TARP member, γ-8, although it increased immunoreactivity in cell bodies in hippocampus, an effect that was not blocked by calpain inhibition. These results strongly suggest the involvement of calpain in the regulation of AMPA receptor targeting and function through truncation of stargazin. Article en texte intégral | | 21256931
 |
PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down.
Sun, Q; Turrigiano, GG
The Journal of neuroscience : the official journal of the Society for Neuroscience
31
6800-8
2010
Afficher le résumé
Synaptic scaling stabilizes neuronal firing through the homeostatic regulation of postsynaptic strength, but the mechanisms by which chronic changes in activity lead to bidirectional adjustments in synaptic AMPA receptor (AMPAR) abundance are incompletely understood. Furthermore, it remains unclear to what extent scaling up and scaling down use distinct molecular machinery. PSD-95 is a scaffold protein proposed to serve as a binding "slot" that determines synaptic AMPAR content, and synaptic PSD-95 abundance is regulated by activity, raising the possibility that activity-dependent changes in the synaptic abundance of PSD-95 or other membrane-associated guanylate kinases (MAGUKs) drives the bidirectional changes in AMPAR accumulation during synaptic scaling. We found that synaptic PSD-95 and SAP102 (but not PSD-93) abundance were bidirectionally regulated by activity, but these changes were not sufficient to drive homeostatic changes in synaptic strength. Although not sufficient, the PSD-95 MAGUKs were necessary for synaptic scaling, but scaling up and down were differentially dependent on PSD-95 and PSD-93. Scaling down was completely blocked by reduced or enhanced PSD-95, through a mechanism that depended on the PDZ1/2 domains. In contrast, scaling up could be supported by either PSD-95 or PSD-93 in a manner that depended on neuronal age and was unaffected by a superabundance of PSD-95. Together, our data suggest that scaling up and down of quantal amplitude is not driven by changes in synaptic abundance of PSD-95 MAGUKs, but rather that the PSD-95 MAGUKs serve as critical synaptic organizers that use distinct protein-protein interactions to mediate homeostatic accumulation and loss of synaptic AMPAR. | | 21543610
 |
Cornichon-2 modulates AMPA receptor-transmembrane AMPA receptor regulatory protein assembly to dictate gating and pharmacology.
Gill, MB; Kato, AS; Roberts, MF; Yu, H; Wang, H; Tomita, S; Bredt, DS
The Journal of neuroscience : the official journal of the Society for Neuroscience
31
6928-38
2010
Afficher le résumé
Neuronal AMPA receptor complexes comprise a tetramer of GluA pore-forming subunits as well as accessory components, including transmembrane AMPA receptor regulatory proteins (TARPs) and cornichon-2/3 (CNIH-2/3). The mechanisms that control AMPA receptor complex assembly remain unclear. AMPA receptor responses in neurons differ from those in cell lines transfected with GluA plus TARPs γ-8 or γ-7, which show unusual resensitization kinetics and non-native AMPA receptor pharmacologies. Using tandem GluA/TARP constructs to constrain stoichiometry, we show here that these peculiar kinetic and pharmacological signatures occur in channels with four TARP subunits per complex. Reducing the number of TARPs per complex produces AMPA receptors with neuron-like kinetics and pharmacologies, suggesting a neuronal mechanism controls GluA/TARP assembly. Importantly, we find that coexpression of CNIH-2 with GluA/TARP complexes reduces TARP stoichiometry within AMPA receptors. In both rat and mouse hippocampal neurons, CNIH-2 also associates with AMPA receptors on the neuronal surface in a γ-8-dependent manner to dictate receptor pharmacology. In the cerebellum, however, CNIH-2 expressed in Purkinje neurons does not reach the neuronal surface. In concordance, stargazer Purkinje neurons, which express CNIH-2 and γ-7, display AMPA receptor kinetics/pharmacologies that can only be recapitulated recombinantly by a low γ-7/GluA stoichiometry. Together, these data suggest that CNIH-2 modulates neuronal AMPA receptor auxiliary subunit assembly by regulating the number of TARPs within an AMPA receptor complex to modulate receptor gating and pharmacology. | | 21543622
 |
Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling.
Steinmetz, CC; Turrigiano, GG
The Journal of neuroscience : the official journal of the Society for Neuroscience
30
14685-90
2009
Afficher le résumé
Glial tumor necrosis factor-α (TNFα) is essential for scaling up of synapses during prolonged activity blockade, but whether TNFα is an instructive or permissive signal is not known. Here we show in rat cortical neurons that the effects of TNFα and activity blockade are not additive; whereas TNFα increased AMPA quantal amplitude at control synapses, TNFα reduced quantal amplitude at prescaled synapses, demonstrating state-dependent effects of TNFα signaling on the scaling process. Whereas synaptic scaling during prolonged activity blockade [24 h tetrodotoxin (TTX)] was prevented by blocking TNFα signaling, early scaling (6 h TTX) was not, unless TNFα signaling was first blocked for 24 h. Moreover, when synapses were prescaled, prolonged (24 h) but not brief (6 h) blockade of TNFα signaling reversed scaling. Finally, prolonged block of TNFα signaling modified the synaptic localization of several scaffold proteins, suggesting that maintenance of postsynaptic density composition is TNFα dependent. Together, these data suggest that TNFα is not an instructive signal for scaling but rather is critical for maintaining synapses in a plastic state in which synaptic scaling can be expressed. | | 21048125
 |
New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors.
Kato, AS; Zhou, W; Milstein, AD; Knierman, MD; Siuda, ER; Dotzlaf, JE; Yu, H; Hale, JE; Nisenbaum, ES; Nicoll, RA; Bredt, DS
The Journal of neuroscience : the official journal of the Society for Neuroscience
27
4969-77
2007
Afficher le résumé
AMPA-type glutamate receptors (GluRs) mediate most excitatory signaling in the brain and are composed of GluR principal subunits and transmembrane AMPA receptor regulatory protein (TARP) auxiliary subunits. Previous studies identified four mammalian TARPs, gamma-2 (or stargazin), gamma-3, gamma-4, and gamma-8, that control AMPA receptor trafficking, gating, and pharmacology. Here, we explore roles for the homologous gamma-5 and gamma-7 proteins, which were previously suggested not to serve as TARPs. Western blotting reveals high levels of gamma-5 and gamma-7 in the cerebellum, where gamma-7 is enriched in Purkinje neurons in the molecular layer and glomerular synapses in the granule cell layer. Immunoprecipitation proteomics shows that cerebellar gamma-7 avidly and selectively binds to AMPA receptor GluR subunits and also binds to the AMPA receptor clustering protein, postsynaptic density-95 (PSD-95). Furthermore, gamma-7 occurs together with PSD-95 and AMPA receptor subunits in purified postsynaptic densities. In heterologous cells, gamma-7 but not gamma-5 greatly enhances AMPA receptor glutamate-evoked currents and modulates channel gating. In granule cells from stargazer mice, transfection of gamma-7 but not gamma-5 increases AMPA receptor-mediated currents. Compared with stargazin, gamma-7 differentially modulates AMPA receptor glutamate affinity and kainate efficacy. These studies define gamma-7 as a new member of the TARP family that can differentially influence AMPA receptors in cerebellar neurons. | | 17475805
 |
Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain.
Deng, F; Price, MG; Davis, CF; Mori, M; Burgess, DL
The Journal of neuroscience : the official journal of the Society for Neuroscience
26
7875-84
2005
Afficher le résumé
The spatial coordination of neurotransmitter receptors with other postsynaptic signaling and structural molecules is regulated by a diverse array of cell-specific scaffolding proteins. The synaptic trafficking of AMPA receptors by the stargazin protein in some neurons, for example, depends on specific interactions between the C terminus of stargazin and the PDZ [postsynaptic density-95 (PSD-95)/Discs large/zona occludens-1] domains of membrane-associated guanylate kinase scaffolding proteins PSD-93 or PSD-95. Stargazin [Cacng2 (Ca2+ channel gamma2 subunit)] is one of four closely related proteins recently categorized as transmembrane AMPA receptor regulating proteins (TARPs) that appear to share similar functions but exhibit distinct expression patterns in the CNS. We used yeast two-hybrid screening to identify MAGI-2 (membrane associated guanylate kinase, WW and PDZ domain containing 2) as a novel candidate interactor with the cytoplasmic C termini of the TARPs. MAGI-2 [also known as S-SCAM (synaptic scaffolding molecule)] is a multi-PDZ domain scaffolding protein that interacts with several different ligands in brain, including PTEN (phosphatase and tensin homolog), dasm1 (dendrite arborization and synapse maturation 1), dendrin, axin, beta- and delta-catenin, neuroligin, hyperpolarization-activated cation channels, beta1-adrenergic receptors, and NMDA receptors. We confirmed that MAGI-2 coimmunoprecipitated with stargazin in vivo from mouse cerebral cortex and used in vitro assays to localize the interaction to the C-terminal -TTPV amino acid motif of stargazin and the PDZ1, PDZ3, and PDZ5 domains of MAGI-2. Expression of stargazin recruited MAGI-2 to cell membranes and cell-cell contact sites in transfected HEK-293T cells dependent on the presence of the stargazin -TTPV motif. These experiments identify MAGI-2 as a strong candidate for linking TARP/AMPA receptor complexes to a wide range of other postsynaptic molecules and pathways and advance our knowledge of protein interactions at mammalian CNS synapses. | | 16870733
 |
The alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptor trafficking regulator "stargazin" is related to the claudin family of proteins by Its ability to mediate cell-cell adhesion.
Price, Maureen G, et al.
J. Biol. Chem., 280: 19711-20 (2005)
2004
Afficher le résumé
Mutations in the Cacng2 gene encoding the neuronal transmembrane protein stargazin result in recessively inherited epilepsy and ataxia in "stargazer" mice. Functional studies suggest a dual role for stargazin, both as a modulatory gamma subunit for voltage-dependent calcium channels and as a regulator of post-synaptic membrane targeting for alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (AMPA)-type glutamate receptors. Co-immunoprecipitation experiments demonstrate that stargazin can bind proteins of either complex in vivo, but it remains unclear whether it can associate with both complexes simultaneously. Cacng2 is one of eight closely related genes (Cacng1-8) encoding proteins with four transmembrane segments, cytoplasmic termini, and molecular masses between 25 and 44 kDa. This group of Cacng genes constitutes only one branch of a larger monophyletic assembly dominated by over 20 genes encoding proteins known as claudins. Claudins regulate cell adhesion and paracellular permeability as fundamental components of non-neuronal tight junctions. Because stargazin is structurally similar to claudins, we hypothesized that it might also have retained claudin-like functions inherited from a common ancestor. Here, we report that expression of stargazin in mouse L-fibroblasts results in cell aggregation comparable with that produced by claudins, and present evidence that the interaction is heterotypic and calcium dependent. The data suggest that the cell adhesion function of stargazin preceded its current role in neurons as a regulator of either voltage-dependent calcium channels or AMPA receptors. We speculate these complexes may have co-opted the established presence of stargazin at sites of close cell-cell contact to facilitate their own evolving intercellular signaling functions. | Immunocytochemistry | 15760900
 |
Nonchannel functions of the calcium channel gamma subunit: insight from research on the stargazer mutant.
Qiao, Xiaoxi and Meng, Hongdi
J. Bioenerg. Biomembr., 35: 661-70 (2003)
2003
Afficher le résumé
Voltage-dependent calcium channels (VDCC) are essential regulators of intracellular calcium concentration, which in turn influences a broad spectrum of cellular functions especially in neurons. Identification of several calcium channel mutations as the cause of neurological disorders in human and mouse indicates the importance of the integrity of these channels to neuronal function. Studies of mutant mice, each carrying a disrupted gene of a different VDCC subunit, have revealed many unexpected roles of these molecules and have significantly advanced our knowledge of subunit function in the last few years. This review addresses recent discoveries of the function of the gamma2 subunit, also named stargazin, with special emphasis on roles other than calcium conductance. | | 15000526
 |
Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms.
Chen, L, et al.
Nature, 408: 936-43 (2000)
1999
Afficher le résumé
Stargazer, an ataxic and epileptic mutant mouse, lacks functional AMPA (alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate) receptors on cerebellar granule cells. Stargazin, the mutated protein, interacts with both AMPA receptor subunits and synaptic PDZ proteins, such as PSD-95. The interaction of stargazin with AMPA receptor subunits is essential for delivering functional receptors to the surface membrane of granule cells, whereas its binding with PSD-95 and related PDZ proteins through a carboxy-terminal PDZ-binding domain is required for targeting the AMPA receptor to synapses. Expression of a mutant stargazin lacking the PDZ-binding domain in hippocampal pyramidal cells disrupts synaptic AMPA receptors, indicating that stargazin-like mechanisms for targeting AMPA receptors may be widespread in the central nervous system. | | 11140673
 |