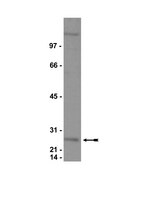
MOZ-mediated repression of p16(INK) (4) (a) is critical for the self-renewal of neural and hematopoietic stem cells.
Perez-Campo, FM; Costa, G; Lie-A-Ling, M; Stifani, S; Kouskoff, V; Lacaud, G
Stem cells (Dayton, Ohio)
32
1591-601
2014
Afficher le résumé
Although inhibition of p16(INK4a) expression is critical to preserve the proliferative capacity of stem cells, the molecular mechanisms responsible for silencing p16(INK4a) expression remain poorly characterized. Here, we show that the histone acetyltransferase (HAT) monocytic leukemia zinc finger protein (MOZ) controls the proliferation of both hematopoietic and neural stem cells by modulating the transcriptional repression of p16(INK4a) . In the absence of the HAT activity of MOZ, expression of p16(INK4a) is upregulated in progenitor and stem cells, inducing an early entrance into replicative senescence. Genetic deletion of p16(INK4a) reverses the proliferative defect in both Moz(HAT) (-) (/) (-) hematopoietic and neural progenitors. Our results suggest a critical requirement for MOZ HAT activity to silence p16(INK4a) expression and to protect stem cells from early entrance into replicative senescence. | 24307508
 |
Developmentally regulated linker histone H1c promotes heterochromatin condensation and mediates structural integrity of rod photoreceptors in mouse retina.
Popova, EY; Grigoryev, SA; Fan, Y; Skoultchi, AI; Zhang, SS; Barnstable, CJ
The Journal of biological chemistry
288
17895-907
2013
Afficher le résumé
Mature rod photoreceptor cells contain very small nuclei with tightly condensed heterochromatin. We observed that during mouse rod maturation, the nucleosomal repeat length increases from 190 bp at postnatal day 1 to 206 bp in the adult retina. At the same time, the total level of linker histone H1 increased reaching the ratio of 1.3 molecules of total H1 per nucleosome, mostly via a dramatic increase in H1c. Genetic elimination of the histone H1c gene is functionally compensated by other histone variants. However, retinas in H1c/H1e/H1(0) triple knock-outs have photoreceptors with bigger nuclei, decreased heterochromatin area, and notable morphological changes suggesting that the process of chromatin condensation and rod cell structural integrity are partly impaired. In triple knock-outs, nuclear chromatin exposed several epigenetic histone modification marks masked in the wild type chromatin. Dramatic changes in exposure of a repressive chromatin mark, H3K9me2, indicate that during development linker histone plays a role in establishing the facultative heterochromatin territory and architecture in the nucleus. During retina development, the H1c gene and its promoter acquired epigenetic patterns typical of rod-specific genes. Our data suggest that histone H1c gene expression is developmentally up-regulated to promote facultative heterochromatin in mature rod photoreceptors. | 23645681
 |
Epigenetic silencing in Friedreich ataxia is associated with depletion of CTCF (CCCTC-binding factor) and antisense transcription.
Irene De Biase,Yogesh K Chutake,Paul M Rindler,Sanjay I Bidichandani
PloS one
4
2009
Afficher le résumé
Over 15 inherited diseases are caused by expansion of triplet-repeats. Friedreich ataxia (FRDA) patients are homozygous for an expanded GAA triplet-repeat sequence in intron 1 of the FXN gene. The expanded GAA triplet-repeat results in deficiency of FXN gene transcription, which is reversed via administration of histone deacetylase inhibitors indicating that transcriptional silencing is at least partially due to an epigenetic abnormality. Article en texte intégral | 19956589
 |
Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence.
Zhang, X; Sejas, DP; Qiu, Y; Williams, DA; Pang, Q
Journal of cell science
120
1572-83
2007
Afficher le résumé
The proinflammatory cytokine tumor necrosis factor alpha (TNFalpha) inhibits hematopoietic stem cell (HSC) expansion, interferes with HSC self-renewal and compromises the ability of HSC to reconstitute hematopoiesis. We have investigated mechanisms by which TNFalpha suppresses hematopoiesis using the genomic instability syndrome Fanconi anemia mouse model deficient for the complementation-group-C gene (Fancc). Examination of senescence makers, such as senescence-associated beta-galactosidase, HP1-gamma, p53 and p16(INK4A) shows that TNFalpha induces premature senescence in bone marrow HSCs and progenitor cells as well as other tissues of Fancc-/- mice. TNFalpha-induced senescence correlates with the accumulation of reactive oxygen species (ROS) and oxidative DNA damage. Neutralization of TNFalpha or deletion of the TNF receptor in Fancc-/- mice (Fancc-/-;Tnfr1-/-) prevents excessive ROS production and hematopoietic senescence. Pretreatment of TNFalpha-injected Fancc-/- mice with a ROS scavenger significantly reduces oxidative base damage, DNA strand breaks and senescence. Furthermore, HSCs and progenitor cells from TNFalpha-treated Fancc-/- mice show increased chromosomal aberrations and have an impaired oxidative DNA-damage repair. These results indicate an intimate link between inflammatory reactive oxygen species and DNA-damage-induced premature senescence in HSCs and progenitor cells, which may play an important role in aging and anemia. | 17405815
 |
Antisense transcription and heterochromatin at the DM1 CTG repeats are constrained by CTCF.
Diane H Cho, Cortlandt P Thienes, Sarah E Mahoney, Erwin Analau, Galina N Filippova, Stephen J Tapscott
Molecular cell
20
483-9
2004
Afficher le résumé
Prior studies of the DM1 locus have shown that the CTG repeats are a component of a CTCF-dependent insulator element and that repeat expansion results in conversion of the region to heterochromatin. We now show that the DM1 insulator is maintained in a local heterochromatin context: an antisense transcript emanating from the adjacent SIX5 regulatory region extends into the insulator element and is converted into 21 nucleotide (nt) fragments with associated regional histone H3 lysine 9 (H3-K9) methylation and HP1gamma recruitment that is embedded within a region of euchromatin-associated H3 lysine 4 (H3-K4) methylation. CTCF restricts the extent of the antisense RNA at the wild-type (wt) DM1 locus and constrains the H3-K9 methylation to the nucleosome associated with the CTG repeat, whereas the expanded allele in congenital DM1 is associated with loss of CTCF binding, spread of heterochromatin, and regional CpG methylation. | 16285929
 |
Oncogene-induced senescence as an initial barrier in lymphoma development.
Melanie Braig, Soyoung Lee, Christoph Loddenkemper, Cornelia Rudolph, Antoine H F M Peters, Brigitte Schlegelberger, Harald Stein, Bernd Dörken, Thomas Jenuwein, Clemens A Schmitt
Nature
436
660-5
2004
Afficher le résumé
Acute induction of oncogenic Ras provokes cellular senescence involving the retinoblastoma (Rb) pathway, but the tumour suppressive potential of senescence in vivo remains elusive. Recently, Rb-mediated silencing of growth-promoting genes by heterochromatin formation associated with methylation of histone H3 lysine 9 (H3K9me) was identified as a critical feature of cellular senescence, which may depend on the histone methyltransferase Suv39h1. Here we show that Emicro-N-Ras transgenic mice harbouring targeted heterozygous lesions at the Suv39h1, or the p53 locus for comparison, succumb to invasive T-cell lymphomas that lack expression of Suv39h1 or p53, respectively. By contrast, most N-Ras-transgenic wild-type ('control') animals develop a non-lymphoid neoplasia significantly later. Proliferation of primary lymphocytes is directly stalled by a Suv39h1-dependent, H3K9me-related senescent growth arrest in response to oncogenic Ras, thereby cancelling lymphomagenesis at an initial step. Suv39h1-deficient lymphoma cells grow rapidly but, unlike p53-deficient cells, remain highly susceptible to adriamycin-induced apoptosis. In contrast, only control, but not Suv39h1-deficient or p53-deficient, lymphomas senesce after drug therapy when apoptosis is blocked. These results identify H3K9me-mediated senescence as a novel Suv39h1-dependent tumour suppressor mechanism whose inactivation permits the formation of aggressive but apoptosis-competent lymphomas in response to oncogenic Ras. | 16079837
 |
Phosphorylation site mutations in heterochromatin protein 1 (HP1) reduce or eliminate silencing activity.
Zhao, T, et al.
J. Biol. Chem., 276: 9512-8 (2001)
2001
Afficher le résumé
HP1 is an essential heterochromatin-associated protein in Drosophila. HP1 has dosage-dependent effects on the silencing of euchromatic genes that are mislocalized to heterochromatin and is required for the normal expression of at least two heterochromatic genes. HP1 is multiply phosphorylated in vivo, and HP1 hyperphosphorylation is correlated with heterochromatin assembly during development. The purpose of this study was to test whether HP1 phosphorylation modifies biological activity and biochemical properties of HP1. To determine sites of HP1 phosphorylation in vivo and whether phosphorylation affects any biochemical properties of HP1, we expressed Drosophila HP1 in lepidopteran cultured cells using a recombinant baculovirus vector. Phosphopeptides were identified by matrix-assisted laser desorption ionization/time of flight mass spectroscopy; these peptides contain target sites for casein kinase II, protein tyrosine kinase, and PIM-1 kinase. Purified HP1 from bacterial (unphosphorylated) and lepidopteran (phosphorylated) cells has similar secondary structure. Phosphorylation has no effect on HP1 self-association but alters the DNA binding properties of HP1, suggesting that phosphorylation could differentially regulate HP1-dependent interactions. Serine-to-alanine and serine-to-glutamate substitutions at consensus protein kinase motifs resulted in reduction or loss of silencing activity of mutant HP1 in transgenic flies. These results suggest that dynamic phosphorylation/dephosphorylation regulates HP1 activity in heterochromatic silencing. | 11121421
 |
The HP1 protein family: getting a grip on chromatin.
Eissenberg, J C and Elgin, S C
Curr. Opin. Genet. Dev., 10: 204-10 (2000)
1999
Afficher le résumé
HP1 was first described in Drosophila as a heterochromatin-associated protein with dosage-dependent effects on heterochromatin-induced gene silencing. Recently, membership of the HP1 protein family has expanded tremendously. A number of intriguing interactions between HP1 and other proteins have been described, implicating HP1 in gene regulation, DNA replication, and nuclear architecture. | 10753776
 |
Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei.
Dignam, J D, et al.
Nucleic Acids Res., 11: 1475-89 (1983)
1982
Afficher le résumé
We have developed a procedure for preparing extracts from nuclei of human tissue culture cells that directs accurate transcription initiation in vitro from class II promoters. Conditions of extraction and assay have been optimized for maximum activity using the major late promoter of adenovirus 2. The extract also directs accurate transcription initiation from other adenovirus promoters and cellular promoters. The extract also directs accurate transcription initiation from class III promoters (tRNA and Ad 2 VA). | 6828386
 |