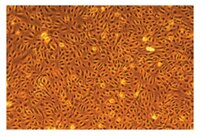
SCCE001 Sigma-AldrichEndoGROTM Human Umbilical Vein Endothelial Cells (HUVEC)
The EndoGRO Human Umbilical Vein Endothelial Cells (HUVEC) are a commonly studied human cell type extracted from human neonatal umbilical cords.
More>> The EndoGRO Human Umbilical Vein Endothelial Cells (HUVEC) are a commonly studied human cell type extracted from human neonatal umbilical cords. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Key Applications |
|---|
| CULT |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Source | Human Neonatal Umbilical Cords |
| Stem Cell Type |
|
| Cell Line Type |
|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 1 vial (500,000 cells) |
| Material Package | 500,000 cells |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| SCCE001 | 04053252576911 |
Documentation
EndoGROTM Human Umbilical Vein Endothelial Cells (HUVEC) SDS
| Title |
|---|
EndoGROTM Human Umbilical Vein Endothelial Cells (HUVEC) Certificates of Analysis
Brochure
| Title |
|---|
| EpiGRO™, EndoGRO™ and FibroGRO™ Reagents |
Technical Info
Data Sheet
| Title |
|---|
| EndoGRO Human Umbilical Vein Endothelial Cells (HUVEC) |