239820 Sigma-AldrichCXCR4 Antagonist I, AMD3100 - CAS 155148-31-5 - Calbiochem
CXCR4 Antagonist I, AMD3100, CAS 155148-31-5, is an antagonist of CXCL12 (SDF1) binding to CXCR4 (IC50 = 108 nM for rat CXCR4) and inhibits SDF1-induced Ca2+ flux.
More>> CXCR4 Antagonist I, AMD3100, CAS 155148-31-5, is an antagonist of CXCL12 (SDF1) binding to CXCR4 (IC50 = 108 nM for rat CXCR4) and inhibits SDF1-induced Ca2+ flux. Less<<Synonyms: 1,1ʹ-(1,4-Phenylenebis(methylene))-bis-1,4,8,11-tetraazacyclotetradecane, 8HCl, JM3100
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 155148-31-5 | C₂₈H₅₄N₈ • 8HCl |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 239820-5MG |
|
5 mg |
|
— |
| Description | |
|---|---|
| Overview | A symmetrical bicyclam compound that antagonizes CXCL12 (SDF1) binding to CXCR4 (IC50 = 108 and 245 nM using rat and human CXCR4, respectively) and inhibits SDF1-induced Ca2+ flux (by 100% at 100 ng/ml in SUP-T1 and THP-1 cultures) as well as CXCR4-, but not CCR5-, mediated HIV infection (IC50 ≤200 nM), while enhancing the binding of SDF1 to CXCR7 (by ~60% at 1 mM with CXCR7-expressing HEK293T cells) and SDF1-induced β-arrestin recruitment to CXCR7 (EC50 = 6.48 and 11.8 nM, in the presence and absence of 10 µM AMD3100, respectively). Administration AMD3100 via intravenous infusion is also reported to result in hematopoietic stem cell mobilization in humans, dogs, and mice in vivo. Exhibits no inhibitory effects against chemokine-induced signaling via CXCR1/2/3 or CCR1/2/3/4/5/6/7/8/9. Also available as a 50 mM solution in H2O (Cat. No. 239825). |
| Catalogue Number | 239820 |
| Brand Family | Calbiochem® |
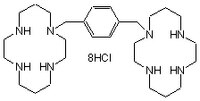
| Synonyms | 1,1ʹ-(1,4-Phenylenebis(methylene))-bis-1,4,8,11-tetraazacyclotetradecane, 8HCl, JM3100 |
| Product Information | |
|---|---|
| CAS number | 155148-31-5 |
| Form | Off-white solid |
| Hill Formula | C₂₈H₅₄N₈ • 8HCl |
| Chemical formula | C₂₈H₅₄N₈ • 8HCl |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | CXCR4 Antagonist I, AMD3100, CAS 155148-31-5, is an antagonist of CXCL12 (SDF1) binding to CXCR4 (IC50 = 108 nM for rat CXCR4) and inhibits SDF1-induced Ca2+ flux. |
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 239820-5MG | 04055977198942 |
Documentation
CXCR4 Antagonist I, AMD3100 - CAS 155148-31-5 - Calbiochem SDS
| Title |
|---|
CXCR4 Antagonist I, AMD3100 - CAS 155148-31-5 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 239820 |
References
| Reference overview |
|---|
| Kalatskaya, I., et al. 2009. Mol. Pharmacol. 77, 1240. Pitchford, S.C., et al. 2009. Cell Stem Cell 4, 62. De Clercq, E. 2009. Biochem. Pharmacol. , 1655. Thoma, G., et al. 2008. J. Med. Chem. 51, 7915. De Clercq, E. 2000. Mol. Pharmacol. 57, 833. |