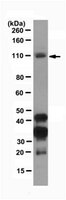
The USP7/Dnmt1 complex stimulates the DNA methylation activity of Dnmt1 and regulates the stability of UHRF1.
Felle, Max, et al.
Nucleic Acids Res., 39: 8355-65 (2011)
2011
Show Abstract
Aberrant DNA methylation is often associated with cancer and the formation of tumors; however, the underlying mechanisms, in particular the recruitment and regulation of DNA methyltransferases remain largely unknown. In this study, we identified USP7 as an interaction partner of Dnmt1 and UHRF1 in vivo. Dnmt1 and USP7 formed a soluble dimer complex that associated with UHRF1 as a trimeric complex on chromatin. Complex interactions were mediated by the C-terminal domain of USP7 with the TS-domain of Dnmt1, whereas the TRAF-domain of USP7 bound to the SRA-domain of UHRF1. USP7 was capable of targeting UHRF1 for deubiquitination and affects UHRF1 protein stability in vivo. Furthermore, Dnmt1, UHRF1 and USP7 co-localized on silenced, methylated genes in vivo. Strikingly, when analyzing the impact of UHRF1 and USP7 on Dnmt1-dependent DNA methylation, we found that USP7 stimulated both the maintenance and de novo DNA methylation activity of Dnmt1 in vitro. Therefore, we propose a dual role of USP7, regulating the protein turnover of UHRF1 and stimulating the enzymatic activity of Dnmt1 in vitro and in vivo. | 21745816
 |