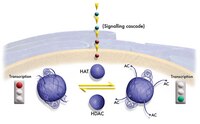
Integrative epigenomic mapping defines four main chromatin states in Arabidopsis.
François Roudier,Ikhlak Ahmed,Caroline Bérard,Alexis Sarazin,Tristan Mary-Huard,Sandra Cortijo,Daniel Bouyer,Erwann Caillieux,Evelyne Duvernois-Berthet,Liza Al-Shikhley,Laurène Giraut,Barbara Després,Stéphanie Drevensek,Frédy Barneche,Sandra Dèrozier,Véronique Brunaud,Sébastien Aubourg,Arp Schnittger,Chris Bowler,Marie-Laure Martin-Magniette,Stéphane Robin,Michel Caboche,Vincent Colot
The EMBO journal
30
2010
Abstract anzeigen
Post-translational modification of histones and DNA methylation are important components of chromatin-level control of genome activity in eukaryotes. However, principles governing the combinatorial association of chromatin marks along the genome remain poorly understood. Here, we have generated epigenomic maps for eight histone modifications (H3K4me2 and 3, H3K27me1 and 2, H3K36me3, H3K56ac, H4K20me1 and H2Bub) in the model plant Arabidopsis and we have combined these maps with others, produced under identical conditions, for H3K9me2, H3K9me3, H3K27me3 and DNA methylation. Integrative analysis indicates that these 12 chromatin marks, which collectively cover ∼90% of the genome, are present at any given position in a very limited number of combinations. Moreover, we show that the distribution of the 12 marks along the genomic sequence defines four main chromatin states, which preferentially index active genes, repressed genes, silent repeat elements and intergenic regions. Given the compact nature of the Arabidopsis genome, these four indexing states typically translate into short chromatin domains interspersed with each other. This first combinatorial view of the Arabidopsis epigenome points to simple principles of organization as in metazoans and provides a framework for further studies of chromatin-based regulatory mechanisms in plants. Volltextartikel | 21487388
 |
Nanobiolistic delivery of indicators to the living mouse retina.
Roberto Roizenblatt,James D Weiland,Stephen Carcieri,Guanting Qiu,Matthew Behrend,Mark S Humayun,Robert H Chow
Journal of neuroscience methods
153
2005
Abstract anzeigen
The development of a technique to load functional indicators into living neurons is an ongoing challenge in retinal neurophysiology. In a number of live-cell preparations, fluorescence-based indicators have been of particular importance for investigating ionic concentrations, protein localization, and other physiological parameters. In the present study, we demonstrate a novel technique that uses a modified gene gun to propel silver nanoparticles coated with indicators into live retinal neurons, and we highlight the advantages of using this technique to deliver these functional indicators. | 16290199
 |
Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics.
Sara A Carney,Hidetoshi Tahara,Carol D Swartz,John I Risinger,Hong He,Alicia B Moore,Joseph K Haseman,J Carl Barrett,Darlene Dixon
Laboratory investigation; a journal of technical methods and pathology
82
2002
Abstract anzeigen
In vitro model systems for studying uterine leiomyomas are limited in that human-derived leiomyoma cells grow poorly in culture compared with normal myometrial cells and begin to senesce early, at approximately passage 10 in our studies. To our knowledge, a good in vitro human-derived cell culturing system for leiomyomas does not exist. In an attempt to fill this void, we have immortalized a uterine leiomyoma cell line by inducing telomerase activity, which allows cells to bypass their normal programmed senescence. Telomerase activity was induced by infecting the target (uterine leiomyoma and normal myometrial) cells with a retroviral vector containing hTERT, the gene for the catalytic subunit of telomerase. Subsequent analysis by RT-PCR and the telomeric repeat amplification protocol assay confirmed expression of the inserted gene and induction of telomerase activity in leiomyoma and myometrial cells. Analysis of cells for estrogen receptor-alpha and progesterone receptor proteins by Western blotting showed no change in expression of these proteins between the immortalized and parental leiomyoma and myometrial cells. Both immortalized and parental myometrial and leiomyoma cells expressed the smooth muscle-specific cytoskeletal protein alpha-actin and were negative for mutant p53 protein as evidenced by immunocytochemical staining. The immortalized leiomyoma and myometrial cells showed no anchorage-independent growth, with the exception of a small subpopulation of immortalized leiomyoma cells at a higher passage that did form two to three small colonies (per 50,000 cells) in soft agar. None of the immortalized cells were tumorigenic in nude mice. In conclusion, our data show the successful insertion of the hTERT gene into leiomyoma and myometrial cells and the immortalization of these cell lines without phenotypic alteration from the parental cell types (up to 200 population doublings). These cells should help to advance research in understanding the molecular pathways involved in the conversion of a normal myometrial cell to a leiomyoma cell and the mechanisms responsible for the growth of uterine leiomyomas. Answers to these questions will undoubtedly lead to the development of more effective treatment and intervention regimens for clinical cases of uterine leiomyoma. | 12065682
 |