346021 Sigma-AldrichGlucokinase Activator, Cpd A - CAS 603108-44-7 - Calbiochem
The Glucokinase Activator, Cpd A, also referenced under CAS 603108-44-7, modulates Glucokinase. This small molecule/inhibitor is primarily used for Activators/Inducers applications.
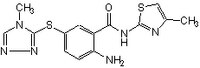
More>> The Glucokinase Activator, Cpd A, also referenced under CAS 603108-44-7, modulates Glucokinase. This small molecule/inhibitor is primarily used for Activators/Inducers applications. Less<<Synonyme: 2-Amino-5-(4-methyl-4H-(1,2,4)-triazole-3-yl-sulfanyl)-N-(4-methyl-thiazole-2-yl)benzamide, Compound A, Hexokinase D Activator, Hexokinase IV Activator
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 603108-44-7 | C₁₄H₁₄N₆OS₂ |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 346021-5MG |
|
Kst.-Ampulle | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 603108-44-7 |
| Form | Off-white solid |
| Hill Formula | C₁₄H₁₄N₆OS₂ |
| Chemical formula | C₁₄H₁₄N₆OS₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information | |
|---|---|
| S Phrase | S: 22-24/25-36 Do not breathe dust. Avoid contact with skin and eyes. Wear suitable protective clothing. |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 346021-5MG | 04055977214628 |
Documentation
Glucokinase Activator, Cpd A - CAS 603108-44-7 - Calbiochem SDB
| Titel |
|---|
Glucokinase Activator, Cpd A - CAS 603108-44-7 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 346021 |
Literatur
| Übersicht |
|---|
| Iino, T., et al. 2009. Bioorg. Med. Chem. Lett. 19, 5531. Mitsuya, M., et al. 2009. Bioorg. Med. Chem. Lett. 19, 2718. Nishimura, T., et al. 2009. Bioorg. Med. Chem. Lett. 19, In press. Futamura, M., et al. 2006. J. Biol. Chem. 281, 1357. Kamata, K., et al. 2004. Structure 12, 429. |