Zell-basierte Assays für Arzneimitteltransport
Millicell-96 cell culture insert plate is a patented 96-well device designed to support epithelial cell growth and differentiation of Caco-2 and other cell lines.
Less<<
Überblick
Spezifikationen
Bestellinformationen
Documentation
Literatur
| Übersicht | Anwendung |
|---|---|
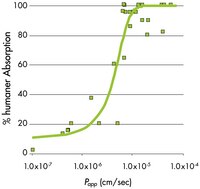
| Use of ADME screening to conserve resources in drug discovery and development Weiss, A. and Joseph Machamer American Biotechnology Laboratory, January 2004: 16-17 2004 | |
| Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. Chisato Yamasaki, Yumiko Natori, Xun-Ting Zeng, Mari Ohmura, Shinji Yamasaki, Yoshifumi Takeda and Yasuhiro Natori FEBS Letters 442 (2-3): 231-234 1998 | Cell Culture |
FAQ
| Frage | Antwort |
|---|---|
| What is the well depth and maximum volume capacity of a MultiScreen plate? | The well depth of a 96 well MultiScreen plate is 1.245 cm. The well depth for a 384 well MultiScreen plate is 1.2 cm. The maximum working volume of a 96 well plate is 300 ul. The maximum working volume for a 384 well plate is 100 ul. |
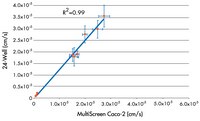
| How do you determine the optimal seeding density on the MultiScreen Caco-2 plate? | This requires optimization for each laboratory, due to the variability in Caco-2 cultures. We recommend starting with the same number of cells/cm2 used for a 24 well culture. For example if you are plating 30,000 cells/well in a 24 well plate, you would plate 11,000 cells per well for the MultiScreen Caco-2 (30,000 cells/0.3cm2)(0.11cm2)= 11,000 cells. |
| Why do we recommend addition of 75 µL of medium apically (in the filter well) and 250 µL basolaterally (in the receiver/feeder plate) for the MultiScreen Caco-2 plate? | These volumes were determined to be optimal for cell growth when feeding the cell culture every other day. In addition, with 75 µL apical, the medium level is at the same height as the basolateral volume of 250 µL. In this way we ensure that there is no positive pressure exerted on the monolayer of Caco-2 cells. |
| Do you recommend a specific way of seeding the cells on a MultiScreen Caco-2 plate? | We recommend pipetting cells at the apical notch and down the side of the well (slow speed if programmable). When seeding many plates, make sure to continually keep cells suspended. |
| Do you recommend optimal feeding conditions for cells grown on the MultiScreen Caco-2 plate? | We recommend feeding every other day (Mon, Wed, Fri.) exchanging apical and basolateral media in a specific order. The preferred order is to aspirate basolaterally first, then apically, and add fresh medium back apically first, then basolaterally. |
| Why is there a defined order for aspirating medium and addition of new medium when feeding the cells on the MultiScreen-Caco-2 plate? | When culturing epithelial cells on filters, it is desirable to limit any pressure the medium may exert which may disrupt the cell contact with the filter. Therefore, the medium in the apical compartment should always be present when medium is present in the basolateral compartment. The preferred order is to aspirate basolaterally first, then apically, and add fresh medium back apically first, then basolaterally. |
| Do you recommend any specific handling when feeding the cells on the MultiScreen-Caco-2 plate? | We recommend using an autoclavable 8-channel aspiration manifold. Sigma M2656. Drummond #3-000-093 Aspirate/feed apically using the apical notch and down the side of the well (slow speed if programmable). If you are feeding manually, feel free to separate the plates to aspirate/feed basolaterally. Due to the fact that the MultiScreen-Caco-2 plate has recessed filter wells, the membrane will not be in direct contact with any surface. The filter plate has 4 feet it stands on. |
| How much does the TEER manual electrode STX-100M cost that is recommended for use with the MultiScreen Caco-2 plate? | The STX-100M probe is $1,200 and you would order this through World Precision Instruments. You will also need a Ohm meter, which is available from Millipore, MERS 000 01. |
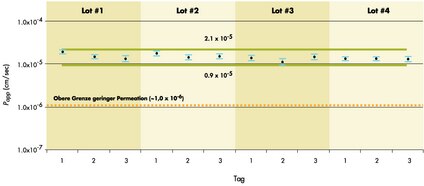
| What are normal TEERs (Trans epithelial electrical resistance) that are measured on the MultiScreen Caco-2 plate? | Generally, TEER values obtained are in the range of 1000-4000 ohms for a 21 day culture and 1000-3000 ohms for a 10 day culture. We consider a failing well below 1000 ohms. If you were using a 24-well plate you can estimate desired TEERs on our plate comparing the surface area. If not, you can estimate a normal range comparing lucifer yellow test results. |
| What lucifer yellow and drugs do you use on the MultiScreen Caco-2 plate? What are your test conditions? | We use lucifer yellow dipotassium salt (LY) from Sigma (L0144) concentration of 100 µg/mL in HBSS). We read LY with a Victor Plate Reader (Wallac/PE). We use tritium labeled drugs spiked with unlabeled drug and use a Microbeta reader (Wallac/PE). We usually run the LY and drug transport experiments for 2 hours, room temperature, shaking at 60 rpm. |