189400 Sigma-AldrichAurintricarboxylic Acid - CAS 4431-00-9 - Calbiochem
A cell-permeable polyanionic, polyaromatic compound used as a powerful inhibitor of cellular processes that are dependent on the formation of protein-nucleic acid complexes.
More>> A cell-permeable polyanionic, polyaromatic compound used as a powerful inhibitor of cellular processes that are dependent on the formation of protein-nucleic acid complexes. Less<<Synonyme: ATA
Empfohlene Produkte
Übersicht
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 4431-00-9 | C₂₂H₁₄O₉ |
Preis & Verfügbarkeit
| Bestellnummer | Verfügbarkeit | Verpackung | St./Pkg. | Preis | Menge | |
|---|---|---|---|---|---|---|
| 189400-100MG |
|
Kst.-Ampulle | 100 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 4431-00-9 |
| ATP Competitive | N |
| Form | Red solid |
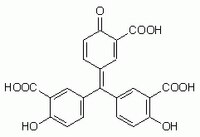
| Hill Formula | C₂₂H₁₄O₉ |
| Chemical formula | C₂₂H₁₄O₉ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | DNA topoisomerase 2 |
| Primary Target IC<sub>50</sub> | 75 nM against DNA topoisomerase II |
| Purity | ≥85% by Titration |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | GU4790000 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Bestellnummer | GTIN |
| 189400-100MG | 04055977221725 |
Documentation
Aurintricarboxylic Acid - CAS 4431-00-9 - Calbiochem SDB
| Titel |
|---|
Aurintricarboxylic Acid - CAS 4431-00-9 - Calbiochem Analysenzertifikate
| Titel | Chargennummer |
|---|---|
| 189400 |
Literatur
| Übersicht |
|---|
| Lozano, R.M., et al. 1997. Eur. J. Biochem. 248, 30. Benchokroun, Y., et al. 1995. Biochem. Pharmacol. 49, 305. Okada, N., and Koizumi, S. 1995. J. Biol. Chem. 270, 16464. Posner, A., et al. 1995. Biochem. Mol. Biol. Int. 36, 291. Catchpoole, D.R., et al. 1994. Anticancer Res. 14, 853. Csernansky, C.A., et al. 1994. J. Neurosci. Res. 38, 101. Gagliardi, A.R., and Collins, D.C. 1994. Anticancer Res. 14, 475. Gonzalez, R.G., et al. 1980. Biochemistry 19, 4299. |
Broschüre
| Titel |
|---|
| Caspases and other Apoptosis Related Tools Brochure |