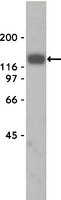
Absence of glial α-dystrobrevin causes abnormalities of the blood-brain barrier and progressive brain edema.
Lien, CF; Mohanta, SK; Frontczak-Baniewicz, M; Swinny, JD; Zablocka, B; Górecki, DC
The Journal of biological chemistry
287
41374-85
2011
Abstract anzeigen
The blood-brain barrier (BBB) plays a key role in maintaining brain functionality. Although mammalian BBB is formed by endothelial cells, its function requires interactions between endotheliocytes and glia. To understand the molecular mechanisms involved in these interactions is currently a major challenge. We show here that α-dystrobrevin (α-DB), a protein contributing to dystrophin-associated protein scaffolds in astrocytic endfeet, is essential for the formation and functioning of BBB. The absence of α-DB in null brains resulted in abnormal brain capillary permeability, progressively escalating brain edema, and damage of the neurovascular unit. Analyses in situ and in two-dimensional and three-dimensional in vitro models of BBB containing α-DB-null astrocytes demonstrated these abnormalities to be associated with loss of aquaporin-4 water and Kir4.1 potassium channels from glial endfeet, formation of intracellular vacuoles in α-DB-null astrocytes, and defects of the astrocyte-endothelial interactions. These caused deregulation of tight junction proteins in the endothelia. Importantly, α-DB but not dystrophins showed continuous expression throughout development in BBB models. Thus, α-DB emerges as a central organizer of dystrophin-associated protein in glial endfeet and a rare example of a glial protein with a role in maintaining BBB function. Its abnormalities might therefore lead to BBB dysfunction. | | | 23043099
 |
Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals.
Kazanis, Ilias, et al.
J. Neurosci., 30: 9771-81 (2010)
2009
Abstract anzeigen
The subependymal zone (SEZ) of the lateral ventricles is one of the areas of the adult brain where new neurons are continuously generated from neural stem cells (NSCs), via rapidly dividing precursors. This neurogenic niche is a complex cellular and extracellular microenvironment, highly vascularized compared to non-neurogenic periventricular areas, within which NSCs and precursors exhibit distinct behavior. Here, we investigate the possible mechanisms by which extracellular matrix molecules and their receptors might regulate this differential behavior. We show that NSCs and precursors proceed through mitosis in the same domains within the SEZ of adult male mice--albeit with NSCs nearer ependymal cells--and that distance from the ventricle is a stronger limiting factor for neurogenic activity than distance from blood vessels. Furthermore, we show that NSCs and precursors are embedded in a laminin-rich extracellular matrix, to which they can both contribute. Importantly, they express differential levels of extracellular matrix receptors, with NSCs expressing low levels of alpha6beta1 integrin, syndecan-1, and lutheran, and in vivo blocking of beta1 integrin selectively induced the proliferation and ectopic migration of precursors. Finally, when NSCs are activated to reconstitute the niche after depletion of precursors, expression of laminin receptors is upregulated. These results indicate that the distinct behavior of adult NSCs and precursors is not necessarily regulated via exposure to differential extracellular signals, but rather via intrinsic regulation of their interaction with their microenvironment. | | | 20660259
 |
Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors.
Patrizi, Annarita, et al.
Proc. Natl. Acad. Sci. U.S.A., 105: 13151-6 (2008)
2008
Abstract anzeigen
GABAergic synapses are crucial for brain function, but the mechanisms underlying inhibitory synaptogenesis are unclear. Here, we show that postnatal Purkinje cells (PCs) of GABA(A)alpha1 knockout (KO) mice express transiently the alpha3 subunit, leading to the assembly of functional GABA(A) receptors and initial normal formation of inhibitory synapses, that are retained until adulthood. Subsequently, down-regulation of the alpha3 subunit causes a complete loss of GABAergic postsynaptic currents, resulting in a decreased rate of inhibitory synaptogenesis and formation of mismatched synapses between GABAergic axons and PC spines. Notably, the postsynaptic adhesion molecule neuroligin-2 (NL2) is correctly targeted to inhibitory synapses lacking GABA(A) receptors and the scaffold molecule gephyrin, but is absent from mismatched synapses, despite innervation by GABAergic axons. Our data indicate that GABA(A) receptors are dispensable for synapse formation and maintenance and for targeting NL2 to inhibitory synapses. However, GABAergic signaling appears to be crucial for activity-dependent regulation of synapse density during neuronal maturation. | Immunofluorescence | Mouse | 18723687
 |
Worldwide distribution and broader clinical spectrum of muscle-eye-brain disease.
Taniguchi, Kiyomi, et al.
Hum. Mol. Genet., 12: 527-34 (2003)
2003
Abstract anzeigen
Muscle-eye-brain disease (MEB), an autosomal recessive disorder prevalent in Finland, is characterized by congenital muscular dystrophy, brain malformation and ocular abnormalities. Since the MEB phenotype overlaps substantially with those of Fukuyama-type congenital muscular dystrophy (FCMD) and Walker-Warburg syndrome (WWS), these three diseases are thought to result from a similar pathomechanism. Recently, we showed that MEB is caused by mutations in the protein O-linked mannose beta1,2-N-acetylglucosaminyltransferase 1 (POMGnT1) gene. We describe here the identification of seven novel disease-causing mutations in six of not only non-Finnish Caucasian but also Japanese and Korean patients with suspected MEB, severe FCMD or WWS. Including six previously reported mutations, the 13 disease-causing mutations we have found thus far are dispersed throughout the entire POMGnT1 gene. We also observed a slight correlation between the location of the mutation and clinical severity in the brain: patients with mutations near the 5' terminus of the POMGnT1 coding region show relatively severe brain symptoms such as hydrocephalus, while patients with mutations near the 3' terminus have milder phenotypes. Our results indicate that MEB may exist in population groups outside of Finland, with a worldwide distribution beyond our expectations, and that the clinical spectrum of MEB is broader than recognized previously. These findings emphasize the importance of considering MEB and searching for POMGnT1 mutations in WWS or other congenital muscular dystrophy patients worldwide. | Immunohistochemistry (Tissue) | | 12588800
 |
Mechanistic distinctions between agrin and laminin-1 induced aggregation of acetylcholine receptors.
Lee, LK; Kunkel, DD; Stollberg, J
BMC neuroscience
3
10
2002
Abstract anzeigen
One of the earliest steps in synaptogenesis at the neuromuscular junction is the aggregation of nicotinic acetylcholine receptors at the postsynaptic membrane. This study presents quantitative analyses of receptor and alpha-Dystroglycan aggregation in response to agrin and laminin-1, alone or in combination.Both laminin and agrin increased overall expression of receptors on the plasma membrane. Following a 24 hour exposure, agrin increased the number of receptor aggregates but did not affect the number of alpha-Dystroglycan aggregates, while the reverse was true of laminin-1. Laminin also increased receptor concentration within aggregates, while agrin had no such effect. Finally, the spatial distribution of aggregates was indistinguishable from random in the case of laminin, while agrin induced aggregates were closer together than predicted by a random model.Agrin and laminin-1 both increase acetylcholine receptor aggregate size after 24 hours, but several lines of evidence indicate that this is achieved via different mechanisms. Agrin and laminin had different effects on the number and density of receptor and alpha-Dystroglycan aggregates. Moreover the random distribution of laminin induced (as opposed to agrin induced) receptor aggregates suggests that the former may influence aggregate size by simple mass action effects due to increased receptor expression. | | | 12182759
 |
Dystroglycan is present in rat thyroid and rat thyroid cells and responds to thyrotropin.
Collins, B J, et al.
Endocrinology, 142: 3152-62 (2001)
2001
Abstract anzeigen
Dystroglycan is a high affinity laminin-binding glycoprotein originally described as a member of the dystrophin-associated glycoprotein complex in muscle. We have demonstrated the presence of dystroglycan in the thyroid using immunocytochemistry, immunoblots, ligand binding assays, and relative quantitative RT-PCR. In intact rat thyroid glands, antibodies against the alpha (extracellular, laminin-binding subunit) and beta (cytoplasmic/membrane bound) portions of the dystroglycan protein reacted at basolateral membranes where they colocalized with laminin. Western-blotted protein from the Fischer rat thyroid cell line FRTL-5 reacted with both the alpha- and beta-dystroglycan antibodies. The alpha-dystroglycan-reactive band colocalized with laminin-binding activity, and the protein and binding activity were decreased by TSH. In contrast, in the culture medium of these cells, alpha-dystroglycan was increased by TSH. The beta-dystroglycan antibody recognized the full-length 43-kDa band and an approximately 30-kDa truncated form. The truncated form was reduced in cells cultured with TSH, whereas the full-length form was not significantly diminished by TSH. Immunofluorescence of FRTL-5 cells in the absence of TSH showed a colocalization of dystroglycan and laminin. This was disrupted by the addition of TSH and was correlated to morphological changes. PCR amplification of complementary DNA with primer pairs from alpha- and beta-dystroglycan produced appropriately sized bands, whose sequence had identical protein-coding sequences and more than 96% nucleotide homology to mouse dystroglycan sequences. Relative quantitative RT-PCR of beta-dystroglycan messenger RNA showed reduced expression in cells cultured with TSH. We conclude that dystroglycan is present in rat thyroid and in FRTL5 rat thyroid cells and that TSH reduces its expression. | Immunocytochemistry, Immunoblotting (Western) | | 11416038
 |
A stoichiometric complex of neurexins and dystroglycan in brain
Sugita, S., et al
J Cell Biol, 154:435-45 (2001)
2001
| Immunocytochemistry, Immunoblotting (Western) | | 11470830
 |
Dystrophic phenotype induced in vitro by antibody blockade of muscle alpha-dystroglycan-laminin interaction
Brown, S. C., et al
J Cell Sci, 112 ( Pt 2):209-16 (1999)
1998
| | | 9858474
 |
MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis.
Widmann, C, et al.
Mol. Cell. Biol., 18: 2416-29 (1998)
1998
Abstract anzeigen
MEK kinase 1 (MEKK1) is a 196-kDa protein that, in response to genotoxic agents, was found to undergo phosphorylation-dependent activation. The expression of kinase-inactive MEKK1 inhibited genotoxin-induced apoptosis. Following activation by genotoxins, MEKK1 was cleaved in a caspase-dependent manner into an active 91-kDa kinase fragment. Expression of MEKK1 stimulated DEVD-directed caspase activity and induced apoptosis. MEKK1 is itself a substrate for CPP32 (caspase-3). A mutant MEKK1 that is resistant to caspase cleavage was impaired in its ability to induce apoptosis. These findings demonstrate that MEKK1 contributes to the apoptotic response to genotoxins. The regulation of MEKK1 by genotoxins involves its activation, which may be part of survival pathways, followed by its cleavage, which generates a proapoptotic kinase fragment able to activate caspases. MEKK1 and caspases are predicted to be part of an amplification loop to increase caspase activity during apoptosis. | Immunoblotting (Western) | | 9528810
 |
Membrane organization of the dystrophin-glycoprotein complex.
Ervasti, J M and Campbell, K P
Cell, 66: 1121-31 (1991)
1991
Abstract anzeigen
The stoichiometry, cellular location, glycosylation, and hydrophobic properties of the components in the dystrophin-glycoprotein complex were examined. The 156, 59, 50, 43, and 35 kd dystrophin-associated proteins each possess unique antigenic determinants, enrich quantitatively with dystrophin, and were localized to the skeletal muscle sarcolemma. The 156, 50, 43, and 35 kd dystrophin-associated proteins contained Asn-linked oligosaccharides. The 156 kd dystrophin-associated glycoprotein contained terminally sialylated Ser/Thr-linked oligosaccharides. Dystrophin, the 156 kd, and the 59 kd dystrophin-associated proteins were found to be peripheral membrane proteins, while the 50 kd, 43 kd, and 35 kd dystrophin-associated glycoproteins and the 25 kd dystrophin-associated protein were confirmed as integral membrane proteins. These results demonstrate that dystrophin and its 59 kd associated protein are cytoskeletal elements that are tightly linked to a 156 kd extracellular glycoprotein by way of a complex of transmembrane proteins. | | | 1913804
 |