344270 Sigma-AldrichForskolin, Coleus forskohlii - CAS 66575-29-9 - Calbiochem
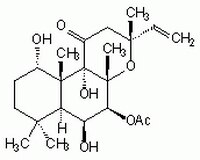
Sinônimos: 7β-Acetoxy-8,13-epoxy-1α,6β,9α-trihydroxy-labd-14-en-11-one, Colforsin
Produtos recomendados
Panorama geral
| Replacement Information |
|---|
Tabela com principais espec.
| CAS # | Empirical Formula |
|---|---|
| 66575-29-9 | C₂₂H₃₄O₇ |
Preço e Disponibilidade
| Número de catálogo | Disponibilidade | Embalagem | Qtde/Emb. | Preço | Quantidade | |
|---|---|---|---|---|---|---|
| 344270-10MG |
|
Ampola plástica | 10 mg |
|
— | |
| 344270-50MG |
|
Ampola plástica | 50 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 66575-29-9 |
| ATP Competitive | N |
| Form | White to off-white crystalline solid |
| Hill Formula | C₂₂H₃₄O₇ |
| Chemical formula | C₂₂H₃₄O₇ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | QL6150000 |
| Safety Information | |
|---|---|
| R Phrase | R: 21 Harmful in contact with skin. |
| S Phrase | S: 36 Wear suitable protective clothing. |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de catálogo | GTIN |
| 344270-10MG | 07790788049294 |
| 344270-50MG | 04055977215366 |
Documentation
Forskolin, Coleus forskohlii - CAS 66575-29-9 - Calbiochem MSDS
| Título |
|---|
Forskolin, Coleus forskohlii - CAS 66575-29-9 - Calbiochem Certificados de análise
| Título | Número do lote |
|---|---|
| 344270 |
Referências
| Visão geral de referência |
|---|
| D'Orazio, J.A., et al. 2006. Nature 443, 340. Noveen, A., et al. 1996. Biochem. Biophys. Res. Commun. 219, 180. Galli, C., et al. 1995. J. Neurosci. 15, 1172. Li, X., et al. 1995. Am. J. Physiol. 269, C986. Lomo, J., et al. 1995. J. Immunol. 154, 1634. Uneyama, H., et al. 1993. J. Biol. Chem. 268, 168. Laurenza, A., et al. 1989. Trends Pharmacol. Sci. 10, 442. Adashi, E.Y., and Resnick, C.E. 1986. J. Cell. Biochem. 31, 217. Seamon, K.B., and Daly, J.W. 1986. Adv. Cyclic Nucleotide Protein Phosphorylation Res. 20, 1. Huang, R., et al. 1982. Cyclic Nucleotide Res. 8, 385. Metzger, H., and Lindner, E. 1981. IRCS Med. Sci. Biochem. Cardiovasc. System Pharmacol. 9, 99. |
Brochura
| Título |
|---|
| Activators and Inhibitors of Adenylate Cyclase Technical Bulletin |
Citações
| Título | |
|---|---|
|
|
| Ficha de dados | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|