239804 Sigma-AldrichCyclopamine-KAAD - Calbiochem
Cyclopamine- KAAD, CAS 306387-90-6, is a cell-permeable potent analog of Cyclopamine (Cat. No. 239803) that specifically inhibits Hedgehog (Hh) signaling with similar or lower toxicity (IC₅₀ = 20 nM).
More>> Cyclopamine- KAAD, CAS 306387-90-6, is a cell-permeable potent analog of Cyclopamine (Cat. No. 239803) that specifically inhibits Hedgehog (Hh) signaling with similar or lower toxicity (IC₅₀ = 20 nM). Less<<Sinônimos: 3-Keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)cyclopamine, KAAD-Cyclopamine, Shh Signaling Antagonist II
Produtos recomendados
Panorama geral
| Replacement Information |
|---|
Tabela com principais espec.
| Empirical Formula |
|---|
| C₄₄H₆₃N₃O₄ |
Preço e Disponibilidade
| Número de catálogo | Disponibilidade | Embalagem | Qtde/Emb. | Preço | Quantidade | |
|---|---|---|---|---|---|---|
| 239804-100UG |
|
Frasco de vidro | 100 μg |
|
— |
| Description | |
|---|---|
| Overview | A potent, cell-permeable analog of Cyclopamine (Cat. No. 239803) that specifically inhibits the Hedgehog (Hh) signaling with similar or lower toxicity (IC50 = 20 nM in Shh-LIGHT2 assay; 50 nM in p2Ptch-/-cells; 500 nM in SmoA1-LIGHT cells). Binds to SmoA1 and promotes its exit from the endoplasmic reticulum. Suppresses both the ShhNp-induced pathway activity and SmoA1-induced reporter activity. Shown to sensitize human glioma cells to TRAIL-induced apoptosis. Also available as a 1 mM solution in DMSO (Cat. No. 239807). |
| Catalogue Number | 239804 |
| Brand Family | Calbiochem® |
| Synonyms | 3-Keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)cyclopamine, KAAD-Cyclopamine, Shh Signaling Antagonist II |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | White to light yellow solid |
| Hill Formula | C₄₄H₆₃N₃O₄ |
| Chemical formula | C₄₄H₆₃N₃O₄ |
| Reversible | N |
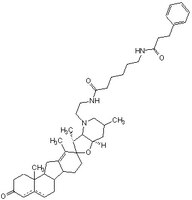
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information | |
|---|---|
| R Phrase | R: 20/21/22 Harmful by inhalation, in contact with skin and if swallowed. |
| S Phrase | S: 22-36/37 Do not breathe dust. Wear suitable protective clothing and gloves. |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de catálogo | GTIN |
| 239804-100UG | 04055977198881 |
Documentation
Cyclopamine-KAAD - Calbiochem MSDS
| Título |
|---|
Cyclopamine-KAAD - Calbiochem Certificados de análise
| Título | Número do lote |
|---|---|
| 239804 |
Referências
| Visão geral de referência |
|---|
| Siegelin, M.D. et al. 2009. Neurobiol. Dis. 34, 259. Watkins, D.N., et al. 2003. Nature 422, 313. Berman, D.M., et al. 2002. Science 297, 1559. Chen, J.K., et al. 2002. Proc. Natl. Acad. Sci. USA 99, 14071. Chen, J.K., et al. 2002. Genes Dev. 16, 2743. Frank-Kamenetsky, M., et al. 2002. J. Biol. 1, 10. Taipale, J., et al. 2000. Nature 406, 1005. |
Ficha de dados
| Título |
|---|
| Reprogramming Cell Fate and Function Novel Strategies for iPSC Generation, Characterization, and Differentiation |
Citações
| Título | |
|---|---|
|
|
| Ficha de dados | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|