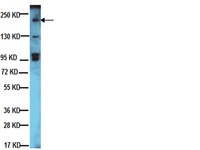
S100A14, a member of the EF-hand calcium-binding proteins, is overexpressed in breast cancer and acts as a modulator of HER2 signaling.
Xu, C; Chen, H; Wang, X; Gao, J; Che, Y; Li, Y; Ding, F; Luo, A; Zhang, S; Liu, Z
The Journal of biological chemistry
289
827-37
2014
Mostrar Resumo
HER2 is overexpressed in 20–25% of breast cancers. Overexpression of HER2 is an adverse prognostic factor and correlates with decreased patient survival. HER2 stimulates breast tumorigenesis via a number of intracellular signaling molecules, including PI3K/AKT and MAPK/ERK.S100A14,one member of the S100 protein family, is significantly associated with outcome of breast cancer patients. Here, for the first time, we show that S100A14 and HER2 are coexpressed in invasive breast cancer specimens,andthere is a significant correlation between the expression levels of the two proteins by immunohistochemistry. S100A14 and HER2 are colocalized in plasma membrane of breast cancer tissue cells and breast cancer cell lines BT474 and SK-BR3. We demonstrate that S100A14 binds directly to HER2 by co-immunoprecipitation and pull-down assays. Further study shows that residues 956–1154 of the HER2 intracellular domain and residue 83 of S100A14 are essential for the two proteins binding.Moreover,we observe a decrease of HER2 phosphorylation, downstream signaling, and HER2-stimulated cell proliferation in S100A14-silenced MCF-7, BT474, and SK-BR3 cells. Our findings suggest that S100A14 functions as a modulator of HER2 signaling and provide mechanistic evidence for its role in breast cancer progression. | Western Blotting | 24285542
 |
Activation of HER3 interferes with antitumor effects of Axl receptor tyrosine kinase inhibitors: suggestion of combination therapy.
Torka, R; Pénzes, K; Gusenbauer, S; Baumann, C; Szabadkai, I; Őrfi, L; Kéri, G; Ullrich, A
Neoplasia (New York, N.Y.)
16
301-18
2014
Mostrar Resumo
The Axl receptor tyrosine kinase (RTK) has been established as a strong candidate for targeted therapy of cancer. However, the benefits of targeted therapies are limited due to acquired resistance and activation of alternative RTKs. Therefore, we asked if cancer cells are able to overcome targeted Axl therapies. Here, we demonstrate that inhibition of Axl by short interfering RNA or the tyrosine kinase inhibitor (TKI) BMS777607 induces the expression of human epidermal growth factor receptor 3 (HER3) and the neuregulin 1(NRG1)-dependent phosphorylation of HER3 in MDA-MB231 and Ovcar8 cells. Moreover, analysis of 20 Axl-expressing cancer cell lines of different tissue origin indicates a low basal phosphorylation of RAC-α serine/threonine-protein kinase (AKT) as a general requirement for HER3 activation on Axl inhibition. Consequently, phosphorylation of AKT arises as an independent biomarker for Axl treatment. Additionally, we introduce phosphorylation of HER3 as an independent pharmacodynamic biomarker for monitoring of anti-Axl therapy response. Inhibition of cell viability by BMS777607 could be rescued by NRG1-dependent activation of HER3, suggesting an escape mechanism by tumor microenvironment. The Axl-TKI MPCD84111 simultaneously blocked Axl and HER2/3 signaling and thereby prohibited HER3 feedback activation. Furthermore, dual inhibition of Axl and HER2/3 using BMS777607 and lapatinib led to a significant inhibition of cell viability in Axl-expressing MDA-MB231 and Ovcar8 cells. Therefore, we conclude that, in patient cohorts with expression of Axl and low basal activity of AKT, a combined inhibition of Axl and HER2/3 kinase would be beneficial to overcome acquired resistance to Axl-targeted therapies. | Western Blotting | 24862757
 |
Metformin and salinomycin as the best combination for the eradication of NSCLC monolayer cells and their alveospheres (cancer stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status.
Xiao, Z; Sperl, B; Ullrich, A; Knyazev, P
Oncotarget
5
12877-90
2014
Mostrar Resumo
The presence of cancer stem cells (CSCs) is linked to preexisting or acquired drug resistance and tumor relapse. Therefore, targeting both differentiated tumor cells and CSCs was suggested as an effective approach for non-small cell lung cancer (NSCLC) treatment. After screening of chemotherapeutic agents, tyrosine kinase inhibitors (TKIs) or monoclonal antibody in combination with the putative stem cell killer Salinomycin (SAL), we found Metformin (METF), which modestly exerted a growth inhibitory effect on monolayer cells and alveospheres/CSCs of 5 NSCLC cell lines regardless of their EGFR, KRAS, EML4/ALK and LKB1 status, interacted synergistically with SAL to effectively promote cell death. Inhibition of EGFR (AKT, ERK1/2) and mTOR (p70 s6k) signaling with the combination of METF and SAL can be augmented beyond that achieved using each agent individually. Phospho-kinase assay further suggested the multiple roles of this combination in reducing oncogenic effects of modules, such as ß-catenin, Src family kinases (Src, Lyn, Yes), Chk-2 and FAK. Remarkably, significant reduction of sphere formation was seen under combinatorial treatment in all investigated NSCLC cell lines. In conclusion, METF in combination with SAL could be a promising treatment option for patients with advanced NSCLC irrespective of their EGFR, KRAS, EML4/ALK and LKB1 status. | Western Blotting | 25375092
 |
Plakoglobin represses SATB1 expression and decreases in vitro proliferation, migration and invasion.
Aktary, Z; Pasdar, M
PloS one
8
e78388
2013
Mostrar Resumo
Plakoglobin (γ-catenin) is a homolog of β-catenin with dual adhesive and signaling functions. Plakoglobin participates in cell-cell adhesion as a component of the adherens junction and desmosomes whereas its signaling function is mediated by its interactions with various intracellular protein partners. To determine the role of plakoglobin during tumorigenesis and metastasis, we expressed plakoglobin in the human tongue squamous cell carcinoma (SCC9) cells and compared the mRNA profiles of parental SCC9 cells and their plakoglobin-expressing transfectants (SCC9-PG). We observed that the mRNA levels of SATB1, the oncogenic chromatin remodeling factor, were decreased approximately 3-fold in SCC9-PG cells compared to parental SCC9 cells. Here, we showed that plakoglobin decreased levels of SATB1 mRNA and protein in SCC9-PG cells and that plakoglobin and p53 associated with the SATB1 promoter. Plakoglobin expression also resulted in decreased SATB1 promoter activity. These results were confirmed following plakoglobin expression in the very low plakoglobin expressing and invasive mammary carcinoma cell line MDA-MB-231 cells (MDA-231-PG). In addition, knockdown of endogenous plakoglobin in the non-invasive mammary carcinoma MCF-7 cells (MCF-7-shPG) resulted in increased SATB1 mRNA and protein. Plakoglobin expression also resulted in increased mRNA and protein levels of the metastasis suppressor Nm23-H1, a SATB1 target gene. Furthermore, the levels of various SATB1 target genes involved in tumorigenesis and metastasis were altered in MCF-7-shPG cells relative to parental MCF-7 cells. Finally, plakoglobin expression resulted in decreased in vitro proliferation, migration and invasion in different carcinoma cell lines. Together with the results of our previous studies, the data suggests that plakoglobin suppresses tumorigenesis and metastasis through the regulation of genes involved in these processes. | | 24260116
 |
ErbB2 dephosphorylation and anti-proliferative effects of neuregulin-1 in ErbB2-overexpressing cells; re-evaluation of their low-affinity interaction.
Wang, R; Iwakura, Y; Araki, K; Keino-Masu, K; Masu, M; Wang, XY; Takei, N; Higashiyama, S; Nawa, H
Scientific reports
3
1402
2013
Mostrar Resumo
Neuregulin-1 binds to ErbB3 and ErbB4 and regulates cancer proliferation and differentiation. Neuregulin-1 had been suggested to also react with ErbB2, but this argument becomes controversial. Here, we re-evaluated the cellular responses and ErbB2 interaction of neuregulin-1 in ErbB2 overexpressing cell lines. In a competitive ligand-binding assay, we detected significant replacement of [(35)S]-labeled neuregulin-1 with nano molar ranges of cold neuregulin-1 in L929 cells expressing ErbB2 alone and SKOV3 cells carrying sulf-1 cDNA but not in these parental cells. The concentration of neuregulin-1 significantly decreased thymidine incorporation and phosphorylation of ErbB2 (Tyr877, Tyr1396, and Tyr1121) in ErbB2-overexpressing cancer cells as well as in L929 cells expressing ErbB2. A crosslinking assay ascertained the presence of neuregulin-1 immunoreactivity in the ErbB2 immune complexes of L929 expressing ErbB2 alone. These results suggest that the higher concentrations of neuregulin-1 exert an anti-oncogenic activity to attenuate ErbB2 auto-phosphorylation potentially through its low-affinity interaction with ErbB2. | | 23466678
 |
A multivariate model of ErbB network composition predicts ovarian cancer cell response to canertinib.
Prasasya RD, Vang KZ, Kreeger PK
Biotechnology and bioengineering
109
213-24. doi
2012
Mostrar Resumo
Identifying the optimal treatment strategy for cancer is an important challenge, particularly for complex diseases like epithelial ovarian cancer (EOC) that are prone to recurrence. In this study we developed a quantitative, multivariate model to predict the extent of ovarian cancer cell death following treatment with an ErbB inhibitor (canertinib, CI-1033). A partial least squares regression model related the levels of ErbB receptors and ligands at the time of treatment to sensitivity to CI-1033. In this way, the model mimics the clinical problem by incorporating only information that would be available at the time of drug treatment. The full model was able to fit the training set data and was predictive. Model analysis demonstrated the importance of including both ligand and receptor levels in this approach, consistent with reports of the role of ErbB autocrine loops in EOC. A reduced multi-protein model was able to predict CI-1033 sensitivity of six distinct EOC cell lines derived from the three subtypes of EOC, suggesting that quantitatively characterizing the ErbB network could be used to broadly predict EOC response to CI-1033. Ultimately, this systems biology approach examining multiple proteins has the potential to uncover multivariate functions to identify subsets of tumors that are most likely to respond to a targeted therapy. Biotechnol. Bioeng. 2012;109: 213-224. © 2011 Wiley Periodicals, Inc.Copyright © 2011 Wiley Periodicals, Inc. | | 21830205
 |
Trastuzumab anti-tumor efficacy in patient-derived esophageal squamous cell carcinoma xenograft (PDECX) mouse models.
Wu, X; Zhang, J; Zhen, R; Lv, J; Zheng, L; Su, X; Zhu, G; Gavine, PR; Xu, S; Lu, S; Hou, J; Liu, Y; Xu, C; Tan, Y; Xie, L; Yin, X; He, D; Ji, Q; Hou, Y; Ge, D
Journal of translational medicine
10
180
2012
Mostrar Resumo
Trastuzumab is currently approved for the clinical treatment of breast and gastric cancer patients with HER-2 positive tumors, but not yet for the treatment of esophageal carcinoma patients, whose tumors typically show 5 ~ 35% HER-2 gene amplification and 0 ~ 56% HER-2 protein expression. This study aimed to investigate the therapeutic efficacy of Trastuzumab in patient-derived esophageal squamous cell carcinoma xenograft (PDECX) mouse models.PDECX models were established by implanting patient esophageal squamous cell carcinoma (ESCC) tissues into immunodeficient (SCID/nude) mice. HER-2 gene copy number (GCN) and protein expression were determined in xenograft tissues and corresponding patient EC samples by FISH and IHC analysis. Trastuzumab anti-tumor efficacy was evaluated within these PDECX models (n = 8 animals/group). Furthermore, hotspot mutations of EGFR, K-ras, B-raf and PIK3CA genes were screened for in the PDECX models and their corresponding patient's ESCC tissues. Similarity between the PDECX models and their corresponding patient's ESCC tissue was confirmed by histology, morphology, HER-2 GCN and mutation.None of the PDECX models (or their corresponding patient's ESCC tissues) harbored HER-2 gene amplification. IHC staining showed HER-2 positivity (IHC 2+) in 2 PDECX models and negativity in 3 PDECX models. Significant tumor regression was observed in the Trastuzumab-treated EC044 HER-2 positive model (IHC 2+). A second HER-2 positive (IHC 2+) model, EC039, harbored a known PIK3CA mutation and showed strong activation of the AKT signaling pathway and was insensitive to Trastuzumab treatment, but could be resensitised using a combination of Trastuzumab and AKT inhibitor AZD5363. In summary, we established 5 PDECX mouse models and demonstrated tumor regression in response to Trastuzumab treatment in a HER-2 IHC 2+ model, but resistance in a HER-2 IHC 2+/PIK3CA mutated model.This study demonstrates Trastuzumab-induced tumor regressions in HER-2 positive tumors, and highlights PIK3CA mutation as a potential resistance mechanism to Trastuzumab treatment in pre-clinical patient-derived EC xenograft models. | | 22935382
 |
Concomitant targeting of EGF receptor, TGF-beta and SRC points to a novel therapeutic approach in pancreatic cancer.
Deharvengt, S; Marmarelis, M; Korc, M
PloS one
7
e39684
2012
Mostrar Resumo
To test the hypothesis that concomitant targeting of the epidermal growth factor receptor (EGFR) and transforming growth factor-beta (TGF-β) may offer a novel therapeutic approach in pancreatic cancer, EGFR silencing by RNA interference (shEGFR) was combined with TGF-β sequestration by soluble TGF-β receptor II (sTβRII). Effects on colony formation in 3-dimensional culture, tumor formation in nude mice, and downstream signaling were monitored. In both ASPC-1 and T3M4 cells, either shEGFR or sTβRII significantly inhibited colony formation. However, in ASPC-1 cells, combining shEGFR with sTβRII reduced colony formation more efficiently than either approach alone, whereas in T3M4 cells, shEGFR-mediated inhibition of colony formation was reversed by sTβRII. Similarly, in vivo growth of ASPC-1-derived tumors was attenuated by either shEGFR or sTβRII, and was markedly suppressed by both vectors. By contrast, T3M4-derived tumors either failed to form or were very small when EGFR alone was silenced, and these effects were reversed by sTβRII due to increased cancer cell proliferation. The combination of shEGFR and sTβRII decreased phospho-HER2, phospho-HER3, phoshpo-ERK and phospho-src (Tyr416) levels in ASPC-1 cells but increased their levels in T3M4 cells. Moreover, inhibition of both EGFR and HER2 by lapatinib or of src by SSKI-606, PP2, or dasatinib, blocked the sTβRII-mediated antagonism of colony formation in T3M4 cells. Together, these observations suggest that concomitantly targeting EGFR, TGF-β, and src may constitute a novel therapeutic approach in PDAC that prevents deleterious cross-talk between EGFR family members and TGF-β-dependent pathways. | | 22761868
 |
HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation.
Takezawa, K; Pirazzoli, V; Arcila, ME; Nebhan, CA; Song, X; de Stanchina, E; Ohashi, K; Janjigian, YY; Spitzler, PJ; Melnick, MA; Riely, GJ; Kris, MG; Miller, VA; Ladanyi, M; Politi, K; Pao, W
Cancer discovery
2
922-33
2012
Mostrar Resumo
EGF receptor (EGFR)-mutant lung cancers eventually become resistant to treatment with EGFR tyrosine kinase inhibitors (TKI). The combination of EGFR-TKI afatinib and anti-EGFR antibody cetuximab can overcome acquired resistance in mouse models and human patients. Because afatinib is also a potent HER2 inhibitor, we investigated the role of HER2 in EGFR-mutant tumor cells. We show in vitro and in vivo that afatinib plus cetuximab significantly inhibits HER2 phosphorylation. HER2 overexpression or knockdown confers resistance or sensitivity, respectively, in all studied cell line models. FISH analysis revealed that HER2 was amplified in 12% of tumors with acquired resistance versus only 1% of untreated lung adenocarcinomas. Notably, HER2 amplification and EGFR(T790M) were mutually exclusive. Collectively, these results reveal a previously unrecognized mechanism of resistance to EGFR-TKIs and provide a rationale to assess the status and possibly target HER2 in EGFR-mutant tumors with acquired resistance to EGFR-TKIs. | Western Blotting | 22956644
 |
Introducing the cancer microenvironment section of Journal of Translational Medicine.
Fernando Vidal-Vanaclocha,Isaac P Witz
Journal of translational medicine
8
2010
Texto completo do artigo | | 20569469
 |