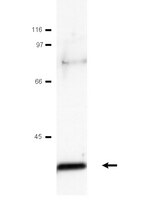
RNA-binding proteins regulate the expression of the immune activating ligand MICB.
Nachmani, D; Gutschner, T; Reches, A; Diederichs, S; Mandelboim, O
Nature communications
5
4186
2014
Mostrar Resumo
The recognition of stress-induced ligands by the activating receptor NKG2D expressed on cytotoxic lymphocytes is crucial for the prevention and containment of various diseases and is also one of the best-studied examples of how danger is sensed by the immune system. Still, however, the mechanisms leading to the expression of the NKG2D ligands are far from being completely understood. Here, we use an unbiased and systematic RNA pull-down approach combined with mass spectrometry to identify six RNA-binding proteins (RBPs) that bind and regulate the expression of MICB, one of the major stress-induced ligands of NKG2D. We further demonstrate that at least two of the identified RBPs function during genotoxic stress. Our data provide insights into stress recognition and hopefully open new therapeutic venues. | Western Blotting | 24924487
 |
The oncogenic microRNA OncomiR-21 overexpressed during Marek's disease lymphomagenesis is transactivated by the viral oncoprotein Meq.
Stik, G; Dambrine, G; Pfeffer, S; Rasschaert, D
Journal of virology
87
80-93
2013
Mostrar Resumo
Gallid herpesvirus 2 (GaHV-2) is an oncogenic herpesvirus that causes T lymphoma in chicken. GaHV-2 encodes a basic leucine zipper (bZIP) protein of the AP-1 family, Meq. Upon formation of homo- or heterodimers with c-Jun, Meq may modulate the expression of viral and cellular genes involved in lymphomagenesis. GaHV-2 also encodes viral microRNAs (miRNAs) involved in latency and apoptosis escape. However, little is known about cellular miRNA deregulation during the development of GaHV-2-associated lymphoma. We determined the cellular miRNA expression profiles of chickens infected with a very virulent strain (RB-1B) or a vaccine strain (CVI988) or noninfected. Among the most deregulated cellular miRNAs, we focused our efforts on gga-miR-21, which is upregulated during GaHV-2 infection. We mapped the gga-miR-21 promoter to the 10th intron of the TMEM49 gene and found it to be driven by AP-1- and Ets-responsive elements. We show here that the viral oncoprotein Meq binds to this promoter, thereby transactivating gga-miR-21 expression. We confirmed that this miRNA targets chicken programmed death cell 4 (PDCD4) and promotes tumor cell growth and apoptosis escape. Finally, gga-miR-21 was overexpressed only during infection with a very virulent strain (RB-1B) and not during infection with a nononcogenic strain (CVI988), providing further evidence for its role in GaHV-2 lymphomagenesis. Our data therefore suggest an additional role for Meq in GaHV-2-mediated lymphomagenesis through the induction of miR-21 expression. | Immunoprecipitation | 23055556
 |
Epigenetic regulation of autophagy by the methyltransferase G9a.
Artal-Martinez de Narvajas, A; Gomez, TS; Zhang, JS; Mann, AO; Taoda, Y; Gorman, JA; Herreros-Villanueva, M; Gress, TM; Ellenrieder, V; Bujanda, L; Kim, DH; Kozikowski, AP; Koenig, A; Billadeau, DD
Mol Cell Biol
33
3983-93
2013
Mostrar Resumo
Macroautophagy is an evolutionarily conserved cellular process involved in the clearance of proteins and organelles. Although the cytoplasmic machinery that orchestrates autophagy induction during starvation, hypoxia, or receptor stimulation has been widely studied, the key epigenetic events that initiate and maintain the autophagy process remain unknown. Here we show that the methyltransferase G9a coordinates the transcriptional activation of key regulators of autophagosome formation by remodeling the chromatin landscape. Pharmacological inhibition or RNA interference (RNAi)-mediated suppression of G9a induces LC3B expression and lipidation that is dependent on RNA synthesis, protein translation, and the methyltransferase activity of G9a. Under normal conditions, G9a associates with the LC3B, WIPI1, and DOR gene promoters, epigenetically repressing them. However, G9a and G9a-repressive histone marks are removed during starvation and receptor-stimulated activation of naive T cells, two physiological inducers of macroautophagy. Moreover, we show that the c-Jun N-terminal kinase (JNK) pathway is involved in the regulation of autophagy gene expression during naive-T-cell activation. Together, these findings reveal that G9a directly represses genes known to participate in the autophagic process and that inhibition of G9a-mediated epigenetic repression represents an important regulatory mechanism during autophagy. | | 23918802
 |
Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells.
Okabe, J; Orlowski, C; Balcerczyk, A; Tikellis, C; Thomas, MC; Cooper, ME; El-Osta, A
Circulation research
110
1067-76
2012
Mostrar Resumo
Epigenetic changes are implicated in the persisting vascular effects of hyperglycemia. The precise mechanism whereby chromatin structure and subsequent gene expression are regulated by glucose in vascular endothelial cells remain to be fully defined.We have studied the molecular and functional mechanism whereby the Set7 methyltransferase associates with chromatin formation and histone methylation in vascular cells in response to current and previous exposure to glucose.To characterize the molecular and functional identity of the Set7 protein, we used vascular cells overexpressing or lacking Set7. Chromatin fractionation for mono-methylation of lysine 4 on histone H3 identified methyltransferase activity. Immunofluorescence experiments strongly suggest that Set7 protein accumulates in the nucleus in response to hyperglycemia. Moreover, activation of proinflammatory genes by high glucose is dependent on Set7 but distinguished by H3K4m1 gene patterns. We show that transient hyperglycemia regulates the expression of proinflammatory genes in vascular endothelial cells in vitro and the persistent increase in glucose-induced gene expression in the aorta of nondiabetic mice.This study uncovers that the response to hyperglycemia in vascular endothelial cells involves the H3K4 methyltransferase, Set7. This enzyme appears to regulate glucose-induced chromatin changes and gene expression not only by H3K4m1-dependent but also H3K4m1-independent pathways. Furthermore, Set7 appears to be responsible for sustained vascular gene expression in response to prior hyperglycemia and is a potential molecular mechanism for the phenomenon of hyperglycemic memory. | Western Blotting | 22403242
 |
Identification of {beta}-catenin binding regions in colon cancer cells using ChIP-Seq.
Bottomly, D; Kyler, SL; McWeeney, SK; Yochum, GS
Nucleic acids research
38
5735-45
2010
Mostrar Resumo
Deregulation of the Wnt/β-catenin signaling pathway is a hallmark of colon cancer. Mutations in the adenomatous polyposis coli (APC) gene occur in the vast majority of colorectal cancers and are an initiating event in cellular transformation. Cells harboring mutant APC contain elevated levels of the β-catenin transcription coactivator in the nucleus which leads to abnormal expression of genes controlled by β-catenin/T-cell factor 4 (TCF4) complexes. Here, we use chromatin immunoprecipitation coupled with massively parallel sequencing (ChIP-Seq) to identify β-catenin binding regions in HCT116 human colon cancer cells. We localized 2168 β-catenin enriched regions using a concordance approach for integrating the output from multiple peak alignment algorithms. Motif discovery algorithms found a core TCF4 motif (T/A-T/A-C-A-A-A-G), an extended TCF4 motif (A/T/G-C/G-T/A-T/A-C-A-A-A-G) and an AP-1 motif (T-G-A-C/T-T-C-A) to be significantly represented in β-catenin enriched regions. Furthermore, 417 regions contained both TCF4 and AP-1 motifs. Genes associated with TCF4 and AP-1 motifs bound β-catenin, TCF4 and c-Jun in vivo and were activated by Wnt signaling and serum growth factors. Our work provides evidence that Wnt/β-catenin and mitogen signaling pathways intersect directly to regulate a defined set of target genes. Texto completo do artigo | | 20460455
 |
Varicella-zoster virus infection of human fibroblast cells activates the c-Jun N-terminal kinase pathway.
Zapata, HJ; Nakatsugawa, M; Moffat, JF
Journal of virology
81
977-90
2007
Mostrar Resumo
The transcription factors ATF-2 and c-Jun are important for transactivation of varicella-zoster virus (VZV) genes. c-Jun is activated by the c-Jun N-terminal kinase (JNK), a member of the mitogen-activated protein kinase pathway that responds to stress and cytokines. To study the effects of VZV on this pathway, confluent human foreskin fibroblasts were infected with cell-associated VZV for 1 to 4 days. Immunoblots showed that phosphorylated JNK and c-Jun levels increased in VZV-infected cells, and kinase assays determined that phospho-JNK was active. Phospho-JNK was detected after 24 h, and levels rose steadily over 4 days in parallel with accumulation of VZV antigen. The two main activators of JNK are MKK4 and MKK7, and levels of their active, phosphorylated forms also increased. The competitive inhibitor of JNK, SP600125, caused a dose-dependent reduction in VZV yield (50% effective concentration, congruent with 8 microM). Specificity was verified by immunoblotting; phospho-c-Jun was eliminated by 18 microM SP600125 in VZV-infected cells. Immunofluorescent confocal microscopy showed that phospho-c-Jun and most of phospho-JNK were in the nuclei of VZV-infected cells; some phospho-JNK was in the cytoplasm. MKK4, MKK7, JNK, and phospho-JNK were detected by immunoblotting in purified preparations of VZV virions, but c-Jun was absent. JNK was located in the virion tegument, as determined by biochemical fractionation and immunogold transmission electron microscopy. Overall, these results demonstrate the importance of the JNK pathway for VZV replication and advance the idea that JNK is a useful drug target against VZV. Texto completo do artigo | | 17079291
 |
The role of activating protein 1 in the transcriptional regulation of the human FCGR2B promoter mediated by the -343 G -> C polymorphism associated with systemic lupus erythematosus.
Olferiev, M; Masuda, E; Tanaka, S; Blank, MC; Pricop, L
The Journal of biological chemistry
282
1738-46
2007
Mostrar Resumo
The inhibitory receptor FcgammaRIIb is a negative regulator of antibody production and inflammatory responses. The -343 G --greater than C polymorphism in the human FCGR2B promoter is associated with systemic lupus erythematosus. The -343 C mutant promoter has decreased transcriptional activity. In the present study, we show that the transcriptional change correlates with quantitative differences in the interaction of the activating protein 1 complex with the mutant FCGR2B promoter. Promoter pulldown and chromatin immunoprecipitation assays demonstrated binding of c-Jun to the FCGR2B promoter. Phosphorylation of c-Jun was accompanied by transactivation of both FCGR2B promoter variants, whereas dephosphorylation of c-Jun by an inhibitor of c-Jun N-terminal kinase, markedly decreased the promoter activities. The -343 G --greater than C substitution enabled the specific interaction of the transcription factor Yin-Yang 1 with the mutant FCGR2B promoter. Yin-Yang 1 competed with activating protein 1 for binding at the -343 site, and contributed to the repression of the mutant FCGR2B promoter activity. This mechanism could be responsible for the decreased expression of FcgammaRIIb associated with the -343 C/C homozygous FCGR2B genotype in lupus patients. These findings provide a rationale for the transcriptional defect mediated by the -343 C/C FCGR2B promoter polymorphism associated with systemic lupus erythematosus, and add to our understanding of the complex transcriptional regulation of the human FCGR2B promoter. | | 17130130
 |
Cytokine activation of p38 mitogen-activated protein kinase and apoptosis is opposed by alpha-4 targeting of protein phosphatase 2A for site-specific dephosphorylation of MEK3.
Prickett, TD; Brautigan, DL
Molecular and cellular biology
27
4217-27
2007
Mostrar Resumo
alpha-4 is an essential gene and is a dominant antiapoptotic factor in various tissues that is a regulatory subunit for type 2A protein phosphatases. A multiplexed phosphorylation site screen revealed that knockdown of alpha-4 by small interfering RNA (siRNA) increased p38 mitogen-activated protein kinase (MAPK) and c-Jun phosphorylation without changes in JNK or ERK. FLAG-alpha-4 coprecipitated hemagglutinin-MEK3 plus endogenous protein phosphatase 2A (PP2A) and selectively enhanced dephosphorylation of Thr193, but not Ser189, in the activation loop of MEK3. Overexpression of alpha-4 suppressed p38 MAPK activation in response to tumor necrosis factor alpha (TNF-alpha). The alpha-4 dominant-negative domain (DND) (residues 220 to 340) associated with MEK3, but not PP2A, and its overexpression sensitized cells to activation of p38 MAPK by TNF-alpha and interleukin-1beta, but not by ansiomycin or sorbitol. The response was diminished by nocodazole or by siRNA knockdown of the Opitz syndrome protein Mid1 that binds alpha-4 to microtubules. Interference by alpha-4 DND or alpha-4 siRNA increased caspase 3/7 activation in response to TNF-alpha. Growth of transformed cells in soft agar was enhanced by alpha-4 and suppressed by alpha-4 DND. The results show that alpha-4 targets PP2A activity to MEK3 to suppress p38 MAPK activation by cytokines, thereby inhibiting apoptosis and anoikis. | Western Blotting | 17438131
 |
WNT pathways in the neonatal ovine uterus: potential specification of endometrial gland morphogenesis by SFRP2.
Hayashi, K; Spencer, TE
Biology of reproduction
74
721-33
2006
Mostrar Resumo
Endometrial glands are critical for uterine function and develop between birth (Postnatal Day [P] 0) and P56 in the neonatal ewe. Endometrial gland morphogenesis or adenogenesis involves the site-specific budding differentiation of the glandular epithelium from the luminal epithelium followed by their coiling/branching development within the stroma of the intercaruncular areas of the endometrium. To determine whether WNT signaling regulates endometrial adenogenesis, the WNT signaling system was studied in the neonatal ovine uterus. WNT5A, WNT7A, and WNT11 were expressed in the uterine epithelia, whereas WNT2B was in the stroma. The WNT receptors FZD2 and FZD6 and coreceptor LRP6 were detected in all uterine cells, and FZD6 was particularly abundant in the endometrial epithelia. Secreted FZD-related protein-2 (SFRP2), a WNT antagonist, was not detected in the P0 uterus, but was abundant in the aglandular caruncular areas of the endometrium between P7 and P56. Exposure of ewes to estrogens during critical developmental periods inhibits or retards endometrial adenogenesis. Estrogen-induced disruption of endometrial adenogenesis was associated with reduction or ablation of WNT2B, WNT7A, and WNT11, and with an increase in WNT2 and SFRP2 mRNA, depending on exposure period. Collectively, results implicate the canonical and noncanonical WNT pathways in regulation of postnatal ovine uterine development and endometrial adenogenesis. Expression of SFRP2 in aglandular caruncular areas may inhibit the WNT signaling pathway, thereby concentrating WNT signaling and restricting endometrial adenogenesis in the intercaruncular areas of the uterus. Further, estrogen-induced inhibition of adenogenesis may be mediated by a reduction in WNT signaling caused by aberrant induction of SFRP2 and loss of several critical WNTs. | Immunohistochemistry (Paraffin) | 16407498
 |
Characterization of the chromosomal binding sites and dimerization partners of the viral oncoprotein Meq in Marek's disease virus-transformed T cells.
Alon M Levy, Yoshihiro Izumiya, Peter Brunovskis, Liang Xia, Mark S Parcells, Sanjay M Reddy, Lucy Lee, Hong-Wu Chen, Hsing-Jien Kung
Journal of virology
77
12841-51
2003
Mostrar Resumo
Marek's disease virus (MDV) is an acute transforming alphaherpesvirus that causes T-cell lymphomas in chickens. We previously reported the identification of a putative oncogene, meq, that is encoded only by the oncogenic serotype of MDV. The gene product, Meq, is a latent protein that is consistently expressed in MDV-transformed lymphoblastoid cells and tumor cells. Meq has a bZIP (basic leucine zipper) structure resembling the family of Jun/Fos. The mechanism whereby Meq transforms T cells remains poorly understood. In this study, we explored the properties of Meq as a transcriptional factor. We analyzed Meq's dimerization partners and its target genes in MSB-1, an MDV-transformed T-cell line. By using in vitro assays, we first demonstrated Meq's potential to dimerize with a variety of bZIP proteins. We then identified c-Jun as the primary dimerization partner of Meq. Both are found to be colocalized in the nucleus and corecruited to promoters with AP-1 sequences. By using chromatin immunoprecipitation (ChIP), we scanned the entire MDV genome for Meq binding sites and found three regions that were enriched with Meq binding: the MDV lytic replication origin, the promoter for Meq, and the promoter for ICP4. Transactivation assays using the above promoters showed that Meq/Meq homodimers exhibited repression activity, whereas Meq/Jun heterodimers showed activation. Finally, we were able to show by ChIP that Meq is recruited to the interleukin-2 promoter in a region encompassing an AP-1 site. Thus, in addition to providing general knowledge about the transcriptional properties of Meq, our studies revealed for the first time the ability of Meq to interact with the latent MDV and host genomes. Our data suggest, therefore, a role for Meq in viral genome regulation during latency, in addition to its putative causal role in T-cell transformation. Texto completo do artigo | | 14610205
 |