Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells.
Patricia F Lalor,Sarah Edwards,Gillian McNab,Marko Salmi,Sirpa Jalkanen,David H Adams
Journal of immunology (Baltimore, Md. : 1950)
169
2002
Mostrar Resumo
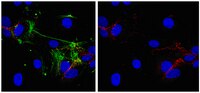
Vascular adhesion protein-1 (VAP-1) is an amine oxidase and adhesion receptor that is expressed by endothelium in the human liver. The hepatic sinusoids are perfused by blood at low flow rates, and sinusoidal endothelium lacks selectin expression and has low levels of CD31, suggesting that VAP-1 may play a specific role in lymphocyte recruitment to the liver. In support of this we now report the constitutive expression of VAP-1 on human hepatic sinusoidal endothelial cells (HSEC) in vitro and demonstrate that VAP-1 supports adhesion and transmigration of lymphocytes across these cells under physiological shear stress. These are the first studies to report the function of VAP-1 on primary human endothelial cells. Under static conditions lymphocyte adhesion to unstimulated HSEC was dependent on VAP-1 and ICAM-2, whereas adhesion to TNF-alpha-stimulated HSEC was dependent on ICAM-1, VCAM-1, and VAP-1. Under conditions of flow, blocking VAP-1 reduced lymphocyte adhesion to TNF-alpha-treated HSEC by 50% and significantly reduced the proportion of adherent lymphocytes that transmigrated across cytokine or LPS-activated endothelium. In addition, inhibition of the amine oxidase activity of VAP-1 reduced both adhesion and transmigration of lymphocytes to a level similar to that seen with VAP-1 Ab. Thus, VAP-1 can support transendothelial migration as well as adhesion, and both functions are dependent on its enzymatic activity. In the absence of selectins and CD31, VAP-1 may play a specific role in lymphocyte recruitment via hepatic sinusoidal endothelium. Moreover, since VAP-1 is induced on nonhepatic endothelium in response to inflammation, its ability to support lymphocyte transendothelial migration may be an important systemic function of VAP-1. | 12097405
 |