538765 Sigma-AldrichPDI Inhibitor IV, LOC14 - Calbiochem
A brain permeant, orally available, reversible, non-covalent nanomolar inhibitor of PDI. Rescues PC12 cells & medium spiny neurons from mutant huntingtin neurotoxicity.
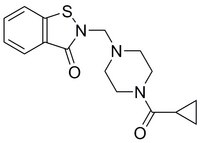
More>> A brain permeant, orally available, reversible, non-covalent nanomolar inhibitor of PDI. Rescues PC12 cells & medium spiny neurons from mutant huntingtin neurotoxicity. Less<<Synonyms: 2-((4-(Cyclopropanecarbonyl)piperazin-1-yl)methyl)benzo[d]isothiazol-3(2H)-one, 2-((4-(Cyclopropylcarbonyl)-1-piperazinyl)methyl)-1,2-benzothiazol-3(2H)-one, Lead Optimized Compound 14, Protein Disulfide Isomerase Inhibitor IV
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₁₆H₁₉N₃O₂S |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5387650001 |
|
Glass bottle | 25 mg |
|
— |
| References | |
|---|---|
| References | Kaplan, A., et al. 2015. Proc. Natl. Acad. Sci. USA 112, In press. |
| Product Information | |
|---|---|
| Form | White solid |
| Hill Formula | C₁₆H₁₉N₃O₂S |
| Chemical formula | C₁₆H₁₉N₃O₂S |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PDI |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5387650001 | 04054839119644 |
Documentation
PDI Inhibitor IV, LOC14 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Kaplan, A., et al. 2015. Proc. Natl. Acad. Sci. USA 112, In press. |