487910 Sigma-Aldrich(±)-S-Nitroso-N-acetylpenicillamine - CAS 79032-48-7 - Calbiochem
Nitric oxide donor that mimics the actions of nitric oxide, including relaxation of isolated bovine coronary artery rings (EC₅₀ = 130 nM).
More>> Nitric oxide donor that mimics the actions of nitric oxide, including relaxation of isolated bovine coronary artery rings (EC₅₀ = 130 nM). Less<<Synonyms: SNAP
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
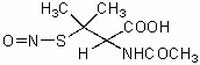
| 79032-48-7 | C₇H₁₂N₂O₄S |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 487910-20MG |
|
Plastic ampoule | 20 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 79032-48-7 |
| ATP Competitive | N |
| Form | Pale green solid |
| Hill Formula | C₇H₁₂N₂O₄S |
| Chemical formula | C₇H₁₂N₂O₄S |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Nitric oxide donor |
| Primary Target IC<sub>50</sub> | EC50 = 130 nM in relaxation of isolated bovine coronary artery rings |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 487910-20MG | 04055977200331 |
Documentation
(±)-S-Nitroso-N-acetylpenicillamine - CAS 79032-48-7 - Calbiochem SDS
| Title |
|---|
(±)-S-Nitroso-N-acetylpenicillamine - CAS 79032-48-7 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 487910 |
References
| Reference overview |
|---|
| Nishio, E., et al. 1996. Biochem. Biophys. Res. Commun. 221, 163. Askew, S.C., et al. 1995. Bioorg. Med. Chem. 3, 1. Fehsel, K., et al. 1995. J. Immunol. 155, 2858. Gopalkrishna, R., et al. 1993. J. Biol. Chem. 268, 27180. Southan, E., and Garthwaite, J. 1991. Neurosci. Lett. 130, 107. Henry, P.J., et al. 1989. J. Pharmacol. Exp. Ther. 248, 762. |
Brochure
| Title |
|---|
| Caspases and other Apoptosis Related Tools Brochure |
Citations
| Title | |
|---|---|
|
|