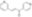525330 Sigma-AldrichPFKFB3 Inhibitor, 3PO - Calbiochem
PFKFB3 Inhibitor, 3PO, is a cell-permeable, selective inhibitor of PFK-2 (6-phosphofructo-2-Kinase) activity.
More>> PFKFB3 Inhibitor, 3PO, is a cell-permeable, selective inhibitor of PFK-2 (6-phosphofructo-2-Kinase) activity. Less<<Synonymes: (2E)-3-Pyridin-3-yl-1-pyridin-4-ylprop-2-en-1-one, 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one, 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 Inhibitor, 3PO
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| Empirical Formula |
|---|
| C₁₃H₁₀N₂O |
Products
| Référence | Conditionnement | Qté | |
|---|---|---|---|
| 525330-25MG | Flacon en verre | 25 mg |
| References | |
|---|---|
| References | Clem, B., et al. 2008. Mol. Cancer Ther. 7, 110. |
| Product Information | |
|---|---|
| Form | Pale yellow solid |
| Hill Formula | C₁₃H₁₀N₂O |
| Chemical formula | C₁₃H₁₀N₂O |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥99% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 525330-25MG | 04055977270716 |
Documentation
PFKFB3 Inhibitor, 3PO - Calbiochem FDS
| Titre |
|---|
PFKFB3 Inhibitor, 3PO - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 525330 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Clem, B., et al. 2008. Mol. Cancer Ther. 7, 110. |