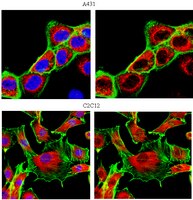
Pitx2a expression alters actin-myosin cytoskeleton and migration of HeLa cells through Rho GTPase signaling.
Wei, Qize and Adelstein, Robert S
Mol. Biol. Cell, 13: 683-97 (2002)
2002
Afficher le résumé
We ectopically expressed the transcription factor Pitx2a, one of the Pitx2 isoforms, in HeLa cells by using a tetracycline-inducible expression system and examined whether Pitx2a was capable of modulating Rho GTPase signaling and altering the cell's cytoskeleton. Ectopic expression of Pitx2a induced actin-myosin reorganization, leading to increased cell spreading, suppression of cell migration, and the strengthening of cell-cell adhesion, marked by the accumulation and localization of beta-catenin and N-cadherin to the sites of cell-cell contacts. Moreover, Pitx2a expression resulted in activation of the Rho GTPases Rac1 and RhoA, and the dominant negative Rac1 mutant N17Rac1 inhibited cell spreading and disrupted localization of beta-catenin to the sites of cell-cell contacts. Both reorganization of actin-myosin and cell spreading require phosphatidylinositol 3-kinase activity, which is also necessary for activation of the Rho GTPase proteins. Pitx2a induced the expression of Trio, a guanine nucleotide exchange factor for Rac1 and RhoA, which preceded cell spreading, and the expression of Trio protein was down-regulated after the changes in cell spreading and cell morphology were initiated. In addition, Pitx2a also induces cell cycle arrest at G0/G1, most likely due to the accumulation of the tumor suppressor proteins p53 and p21. Our data indicate that the transcriptional activities initiated in the nucleus by Pitx2a result in profound changes in HeLa cell morphology, migration, and proliferation. | Activation Assay | 11854422
 |
PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo.
Alessi, D R, et al.
J. Biol. Chem., 270: 27489-94 (1995)
1994
Afficher le résumé
PD 098059 has been shown previously to inhibit the dephosphorylated form of mitogen-activated protein kinase kinase-1 (MAPKK1) and a mutant MAPKK1(S217E,S221E), which has low levels of constitutive activity (Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J., and Saltiel, A. R. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7686-7689). Here we report that PD 098059 does not inhibit Raf-activated MAPKK1 but that it prevents the activation of MAPKK1 by Raf or MEK kinase in vitro at concentrations (IC50 = 2-7 microM) similar to those concentrations that inhibit dephosphorylated MAPKK1 or MAPKK1(S217E,S221E). PD 098059 inhibited the activation of MAPKK2 by Raf with a much higher IC50 value (50 microM) and did not inhibit the phosphorylation of other Raf or MEK kinase substrates, indicating that it exerts its effect by binding to the inactive form of MAPKK1. PD 098059 also acts as a specific inhibitor of the activation of MAPKK in Swiss 3T3 cells, suppressing by 80-90% its activation by a variety of agonists. The high degree of specificity of PD 098059 in vitro and in vivo is indicated by its failure to inhibit 18 protein Ser/Thr kinases (including two other MAPKK homologues) in vitro by its failure to inhibit the in vivo activation of MAPKK and MAP kinase homologues that participate in stress and interleukin-1-stimulated kinase cascades in KB and PC12 cells, and by lack of inhibition of the activation of p70 S6 kinase by insulin or epidermal growth factor in Swiss 3T3 cells. PD 098059 (50 microM) inhibited the activation of p42MAPK and isoforms of MAP kinase-activated protein kinase-1 in Swiss 3T3 cells, but the extent of inhibition depended on how potently c-Raf and MAPKK were activated by any particular agonist and demonstrated the enormous amplification potential of this kinase cascade. PD 098059 not only failed to inhibit the activation of Raf by platelet-derived growth factor, serum, insulin, and phorbol esters in Swiss 3T3 cells but actually enhanced Raf activity. The rate of activation of Raf by platelet-derived growth factor was increased 3-fold, and the subsequent inactivation that occurred after 10 min was prevented. These results indicate that the activation of Raf is suppressed and that its inactivation is accelerated by a downstream component(s) of the MAP kinase pathway. | | 7499206
 |
A synthetic inhibitor of the mitogen-activated protein kinase cascade.
Dudley, D T, et al.
Proc. Natl. Acad. Sci. U.S.A., 92: 7686-9 (1995)
1994
Afficher le résumé
Treatment of cells with a variety of growth factors triggers a phosphorylation cascade that leads to activation of mitogen-activated protein kinases (MAPKs, also called extracellular signal-regulated kinases, or ERKs). We have identified a synthetic inhibitor of the MAPK pathway. PD 098059 [2-(2'-amino-3'-methoxyphenyl)-oxanaphthalen-4-one] selectively inhibited the MAPK-activating enzyme, MAPK/ERK kinase (MEK), without significant inhibitory activity of MAPK itself. Inhibition of MEK by PD 098059 prevented activation of MAPK and subsequent phosphorylation of MAPK substrates both in vitro and in intact cells. Moreover, PD 098059 inhibited stimulation of cell growth and reversed the phenotype of ras-transformed BALB 3T3 mouse fibroblasts and rat kidney cells. These results indicate that the MAPK pathway is essential for growth and maintenance of the ras-transformed phenotype. Further, PD 098059 is an invaluable tool that will help elucidate the role of the MAPK cascade in a variety of biological settings. | | 7644477
 |
Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor.
Pang, L, et al.
J. Biol. Chem., 270: 13585-8 (1995)
1994
Afficher le résumé
The mitogen-activated protein kinase (MAP kinase) pathway is thought to play an important role in the actions of neurotrophins. A small molecule inhibitor of the upstream kinase activator of MAP kinase, MAP kinase kinase (MEK) was examined for its effect on the cellular action of nerve growth factor (NGF) in PC-12 pheochromocytoma cells. PD98059 selectively blocks the activity of MEK, inhibiting both the phosphorylation and activation of MAP kinases in vitro. Pretreatment of PC-12 cells with the compound completely blocked the 4-fold increase in MAP kinase activity produced by NGF. Half-maximal inhibition was observed at 2 microM PD98059, with maximal effects at 10-100 microM. The tyrosine phosphorylation of immunoprecipitated MAP kinase was also completely blocked by the compound. In contrast, the compound was without effect on NGF-dependent tyrosine phosphorylation of the pp140trk receptor or its substrate Shc and did not block NGF-dependent activation of phosphatidylinositol 3'-kinase. However, PD98059 completely blocked NGF-induced neurite formation in these cells without altering cell viability. These data indicate that the MAP kinase pathway is absolutely required for NGF-induced neuronal differentiation in PC-12 cells. | | 7775407
 |