412512 Sigma-AldrichIRE1 Inhibitor III, 4μ8C - CAS 14003-96-4 - Calbiochem
IRE1 Inhibitor III, , CAS 14003-96-4, is a cell-permeable. Covalent inhibitor of IRE1 RNase activity (IC₅₀ = 550 and 45 nM, respectively, with 0 & 16 min preincubation in RNA cleavage assays).
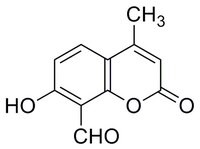
More>> IRE1 Inhibitor III, , CAS 14003-96-4, is a cell-permeable. Covalent inhibitor of IRE1 RNase activity (IC₅₀ = 550 and 45 nM, respectively, with 0 & 16 min preincubation in RNA cleavage assays). Less<<Synonymes: ER-to-Nucleus Signaling 1 Inhibitor III, Inositol-Reguiring Protein 1 Inhibitor III, 8-Formyl-7-hydroxy-4-methylcoumarin, 7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 14003-96-4 | C₁₁H₈O₄ |
Products
| Référence | Conditionnement | Qté | |
|---|---|---|---|
| 412512-25MG | Flacon en verre | 25 mg |
| Description | |
|---|---|
| Overview | A cell-permeable coumarin o-hydroxyaldehyde that inhibits IRE1 RNase activity in a time- and dose-dependent manner (IC50 = 550 and 45 nM, respectively, with 0 and 16 min drug preincubation in RNA cleavage assays) by covalently targeting IRE1 Lys907 via Schiff base formation, effectively preventing ER stress-induced site-specific mRNA splicing as well as RIDD (Regulated IRE1-Dependent Degradation) mRNA degradation (IC50 = 6.9 and 4.1 µM, respectively, against Xbp1 splicing and Scara3 degradation) in MEF cultures following Tunicamycin (Cat. No. 654380) treatment. Comparing to STF083010 (Cat. No. 412510), 4μ8C is also shown to inhibit IRE1 autophosphorylation in cell-free assays via Schiff base formation with IRE1 Lys599 in the absence of ADP, however cellular nucleotide prevents 4μ8C from targeting IRE1 Lys599 intracellularly. |
| Catalogue Number | 412512 |
| Brand Family | Calbiochem® |
| Synonyms | ER-to-Nucleus Signaling 1 Inhibitor III, Inositol-Reguiring Protein 1 Inhibitor III, 8-Formyl-7-hydroxy-4-methylcoumarin, 7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde |
| References | |
|---|---|
| References | Cross, B.C.S., et al. 2012. Proc. Natl. acad. Sci. USA 109, E869. |
| Product Information | |
|---|---|
| CAS number | 14003-96-4 |
| Form | Yellow powder |
| Hill Formula | C₁₁H₈O₄ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | IRE1 |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 412512-25MG | 04055977188189 |
Documentation
IRE1 Inhibitor III, 4μ8C - CAS 14003-96-4 - Calbiochem FDS
| Titre |
|---|
IRE1 Inhibitor III, 4μ8C - CAS 14003-96-4 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 412512 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Cross, B.C.S., et al. 2012. Proc. Natl. acad. Sci. USA 109, E869. |
| Fiche technique | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|