239802 Sigma-AldrichCurcumin, Curcuma longa L. - CAS 458-37-7 - Calbiochem
A cell-permeable and irreversible antitumor and anti-inflammatory agent that acts as an inhibitor of 5-lipoxygenase (IC₅₀ = 8 µM) and cyclooxygenase (IC₅₀ = 52 µM).
More>> A cell-permeable and irreversible antitumor and anti-inflammatory agent that acts as an inhibitor of 5-lipoxygenase (IC₅₀ = 8 µM) and cyclooxygenase (IC₅₀ = 52 µM). Less<<Synonymes: 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, Histone Acetyltransferase Inhibitor I, HAT Inhibitor I, p300/CBP Inhibitor I, NOD2 Signaling Inhibitor I, Nucleotide-binding Oligomerization Domain 2 Signaling Inhibitor I
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
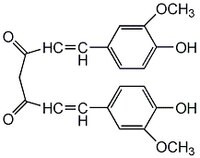
| 458-37-7 | C₂₁H₂₀O₆ |
Products
| Référence | Conditionnement | Qté | |
|---|---|---|---|
| 239802-100MG | Ampoule plast. | 100 mg |
| Product Information | |
|---|---|
| CAS number | 458-37-7 |
| ATP Competitive | N |
| Form | Orange-yellow solid |
| Hill Formula | C₂₁H₂₀O₆ |
| Chemical formula | C₂₁H₂₀O₆ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | MI5230000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 239802-100MG | 04055977199581 |
Documentation
Curcumin, Curcuma longa L. - CAS 458-37-7 - Calbiochem FDS
| Titre |
|---|
Curcumin, Curcuma longa L. - CAS 458-37-7 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 239802 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Hung, S., et al. 2008. Mol. Pharmacol. 74, 274. Cui, L., et al. 2007. Antimicrob. Agents Chemother. 51, 488. Salvioli, S., et al. 2007. eCAM 4, 181. Balasubramanyam, K. et al. 2004. J. Biol. Chem. 279, 51163. Brouet, I., and Okshima, H. 1995. Biochem. Biophys. Res. Commun. 206, 533. Korutla, L., and Kumar, R. 1994. Biochim. Biophys. Acta 1224, 597. Flynn, D.L., et al. 1986. Prostagland. Leuk. Med. 22, 357. |
Brochure
| Titre |
|---|
| Alzheimer's Disease Brochure & Technical Guide |
Citations
| Titre | |
|---|---|
|
|