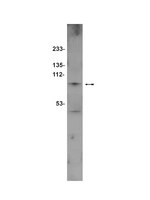
Downregulation of Smurf2, a tumor-suppressive ubiquitin ligase, in triple-negative breast cancers: involvement of the RB-microRNA axis.
Liu, X; Gu, X; Sun, L; Flowers, AB; Rademaker, AW; Zhou, Y; Kiyokawa, H
BMC cancer
14
57
2014
Afficher le résumé
The HECT family ubiquitin ligase Smurf2 regulates cell polarity, migration, division, differentiation and death, by targeting diverse substrates that are critical for receptor signaling, cytoskeleton, chromatin remodeling and transcription. Recent studies suggest that Smurf2 functions as a tumor suppressor in mice. However, no inactivating mutation of SMURF2 has been reported in human, and information about Smurf2 expression in human cancer remains limited or complicated. Here we demonstrate that Smurf2 expression is downregulated in human breast cancer tissues, especially of the triple-negative subtype, and address the mechanism of Smurf2 downregulation in triple-negative breast cancer cells.Human breast cancer tissues (47 samples expressing estrogen receptor (ER) and 43 samples with triple-negative status) were examined by immunohistochemistry for the expression of Smurf2. Ten widely-studied human breast cancer cell lines were examined for the expression of Smurf2. Furthermore, microRNA-mediated regulation of Smurf2 was investigated in triple-negative cancer cell lines.Immunohistochemical analysis showed that benign mammary epithelial cells expressed high levels of Smurf2, so did cells in ductal carcinomas in situ. In contrast, invasive ductal carcinomas showed focal or diffuse decrease in Smurf2 expression, which was observed more frequently in triple-negative tumors than in ER-positive tumors. Consistently, human triple-negative breast cancer cell lines such as BT549, MDA-MB-436, DU-4475 and MDA-MB-468 cells showed significantly lower expression of Smurf2 protein, compared to ER + or HER2+ cell lines. Studies using quantitative PCR and specific microRNA inhibitors indicated that increased expression of miR-15a, miR-15b, miR-16 and miR-128 was involved in Smurf2 downregulation in those triple-negative cancer cell lines, which have mutations in the retinoblastoma (RB) gene. Forced expression of RB increased levels of Smurf2 protein with concomitant decreases in the expression of the microRNAs.This study provides evidence of posttranscriptional downregulation of Smurf2 in triple-negative breast cancers, and demonstrates that the loss of RB function is involved in microRNA-mediated interference with Smurf2 translation. The new link from RB inactivation to Smurf2 downregulation is likely to play a role in malignant phenotypes of triple-negative breast cancer cells. | Western Blotting | 24485087
 |
The E3 ubiquitin ligase SMAD ubiquitination regulatory factor 2 negatively regulates Krüppel-like factor 5 protein.
Du, JX; Hagos, EG; Nandan, MO; Bialkowska, AB; Yu, B; Yang, VW
The Journal of biological chemistry
286
40354-64
2010
Afficher le résumé
The zinc finger transcription factor Krüppel-like factor 5 (KLF5) is regulated posttranslationally. We identified SMAD ubiquitination regulatory factor 2 (SMURF2), an E3 ubiquitin ligase, as an interacting protein of KLF5 by yeast two-hybrid screen, coimmunoprecipitation, and indirect immunofluorescence studies. The SMURF2-interacting domains in KLF5 were mapped to its carboxyl terminus, including the PY motif of KLF5 and its zinc finger DNA-binding domain. KLF5 protein levels were reduced significantly upon overexpression of SMURF2 but not catalytically inactive SMURF2-C716A mutant or SMURF1. SMURF2 alone reduced the protein stability of KLF5 as shown by cycloheximide chase assay, indicating that SMURF2 specifically destabilizes KLF5. In contrast, KLF5(1-165), a KLF5 amino-terminal construct that lacks the PY motif and DNA binding domain, was not degraded by SMURF2. The degradation of KLF5 by SMURF2 was blocked by the proteasome inhibitor MG132, and SMURF2 efficiently ubiquitinated both overexpressed and endogenous KLF5. In contrast, knocking down SMURF2 by siRNAs significantly enhanced KLF5 protein levels, reduced ubiquitination of KLF5, and increased the expression of cyclin D1 and PDGF-A, two established KLF5 target genes. In consistence, SMURF2, but not the E3 ligase mutant SMURF2-C716A, significantly inhibited the transcriptional activity of KLF5, as demonstrated by dual luciferase assay using the PDGF-A promoter, and suppressed the ability of KLF5 to stimulate cell proliferation as measured by BrdU incorporation. Hence, SMURF2 is a novel E3 ubiquitin ligase for KLF5 and negatively regulates KLF5 by targeting it for proteasomal degradation. | Western Blotting | 21953463
 |
Regulation of TGF-beta signalling by Fbxo11, the gene mutated in the Jeff otitis media mouse mutant.
Tateossian, H; Hardisty-Hughes, RE; Morse, S; Romero, MR; Hilton, H; Dean, C; Brown, SD
PathoGenetics
2
5
2009
Afficher le résumé
Jeff is a dominant mouse mutant displaying chronic otitis media. The gene underlying Jeff is Fbxo11, a member of the large F-box family, which are specificity factors for the SCF E3 ubiquitin ligase complex. Jeff homozygotes die shortly after birth displaying a number of developmental abnormalities including cleft palate and eyes open at birth. TGF-beta signalling is involved in a number of epithelial developmental processes and we have investigated the impact of the Jeff mutation on the expression of this pathway.Phospho-Smad2 (pSmad2) is significantly upregulated in epithelia of Jeff homozygotes. Moreover, there was a significant increase in nuclear localization of pSmad2 in contrast to wild type. Mice heterozygous for both Jeff and Smad2 mutations recapitulate many of the features of the Jeff homozygous phenotype. However, tissue immunoprecipitations failed to detect any interaction between Fbxo11 and Smad2. Fbxo11 is known to neddylate p53, a co-factor of pSmad2, but we did not find any evidence of genetic interactions between Jeff and p53 mutants. Nevertheless, p53 levels are substantially reduced in Jeff mice suggesting that Fbxo11 plays a role in stabilizing p53.Overall, our findings support a model whereby Fbxo11, possibly via stabilization of p53, is required to limit the accumulation of pSmad2 in the nucleus of epithelial cells of palatal shelves, eyelids and airways of the lungs. The finding that Fbxo11 impacts upon TGF-beta signalling has important implications for our understanding of the underlying disease mechanisms of middle ear inflammatory disease. | | 19580641
 |
Expression of Smad ubiquitin regulatory factor 2 (Smurf2) in rhesus monkey endometrium and placenta during early pregnancy.
Yang, Q; Lin, HY; Wang, HX; Zhang, H; Zhang, X; Wang, HM; Zhu, C
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society
55
453-60
2007
Afficher le résumé
Smad ubiquitin regulatory factor 2 (Smurf2) is an E3 ubiquitin ligase that is involved in the Smad-mediated TGF-beta signaling. TGF-beta has been shown to play an important role during normal embryo implantation, but whether Smurf2 is involved in this process has not been reported. This study was first conducted to investigate the expression of Smurf2 transcript and protein in different compartments of the rhesus monkey uteri and placenta during early pregnancy. The results showed that both the cloned partial sequence of Smurf2 gene and the corresponding amino acid residues shared 99% identity with those of human homologs. On day 12 (D12) of pregnancy, strong signals of Smurf2 mRNA were found in basalis glandular epithelium and luminal epithelium, and moderate expressions were detected in functionalis glandular epithelium. During early villi stage and villi placental stage, Smurf2 mRNAs were mainly localized in the placenta villi, trophoblastic column, trophoblastic shell, and basalis glandular epithelium. There appeared strong staining signals in the arterioles on D26 of pregnancy, but faint staining signals on D18 of pregnancy. No specific staining of Smurf2 mRNA was observed in stromal cells and myometrium. The expression pattern of Smurf2 protein was generally similar to that of its mRNA. These results provide the first evidence that Smurf2 may play specific roles in glandular secretion, trophoblastic cell invasion, and placentation through mediating the expression of the related proteins of TGF-beta signaling pathway during early pregnancy. | | 17189523
 |
LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads.
Sangadala, S; Boden, SD; Viggeswarapu, M; Liu, Y; Titus, L
The Journal of biological chemistry
281
17212-9
2005
Afficher le résumé
Development and repair of the skeletal system and other organs is highly dependent on precise regulation of bone morphogenetic proteins (BMPs), their receptors, and their intracellular signaling proteins known as Smads. The use of BMPs clinically to induce bone formation has been limited in part by the requirement of much higher doses of recombinant proteins in primates than were needed in cell culture or rodents. Therefore, control of cellular responsiveness to BMPs is now a critical area that is poorly understood. We determined that LMP-1, a LIM domain protein capable of inducing de novo bone formation, interacts with Smurf1 (Smad ubiquitin regulatory factor 1) and prevents ubiquitination of Smads. In the region of LMP responsible for bone formation, there is a motif that directly interacts with the Smurf1 WW2 domain and can effectively compete with Smad1 and Smad5 for binding. We have shown that small peptides containing this motif can mimic the ability to block Smurf1 from binding Smads. This novel interaction of LMP-1 with the WW2 domain of Smurf1 to block Smad binding results in increased cellular responsiveness to exogenous BMP and demonstrates a novel regulatory mechanism for the BMP signaling pathway. | Western Blotting | 16611643
 |