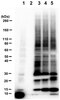
Monocyte chemoattractant protein-1 affects migration of hippocampal neural progenitors following status epilepticus in rats.
Hung, YW; Lai, MT; Tseng, YJ; Chou, CC; Lin, YY
Journal of neuroinflammation
10
11
2013
Afficher le résumé
Epilepsy is a common brain disorder characterized by a chronic predisposition to generate spontaneous seizures. The mechanisms for epilepsy formation remain unknown. A growing body of evidence suggests the involvement of inflammatory processes in epileptogenesis. In the present study, we investigated the involvement of monocyte chemoattractant protein-1 (MCP-1) in aberrant migration of hippocampal progenitors in rats after the insult of status epilepticus (SE).SE was induced with pilocarpine in Sprague-Dawley rats. Transcriptional expression of MCP-1 in the dentate gyrus (DG) was measured using quantitative real-time PCR. From 1 to 28 days after SE, the temporal profiles of MCP-1 protein expression in DG were evaluated using enzyme-linked immunosorbent assay. Chemokine (C-C motif) receptor 2 (CCR2) expression in doublecortin-positive neuronal progenitors was examined using double-labeling immunohistochemistry. The involvement of MCP-1/CCR2 signaling in aberrant neuronal progenitor migration in the epileptic hippocampus was assessed in the SE rats using a CCR2 antagonist, RS102895, and the ectopic migration of neuronal progenitors was determined using Prox1/doublecortin double immunostaining.After SE, MCP-1 gene was significantly upregulated and its corresponding protein expression in the DG was significantly increased on days 1 and 3. Some hilar ectopic progenitor cells of SE rats expressed the MCP-1 receptor, CCR2. Notably, the ectopic migration of neuronal progenitors into hilus was attenuated by a blockade of the MCP-1/CCR2 interaction with a selective CCR2 inhibitor, RS102895.An increase in dentate MCP-1 is associated with seizure-induced aberrant migration of neuronal progenitors through the interaction with CCR2. The upregulation of MCP-1 after an insult of SE may play a role in the generation of epilepsy. | Immunohistochemistry | 23339567
 |
The role of egr1 in early zebrafish retinogenesis.
Zhang, L; Cho, J; Ptak, D; Leung, YF
PloS one
8
e56108
2013
Afficher le résumé
Proper retinal cell differentiation is essential for establishing a functional retina. The purpose of this study is to investigate the role of early growth response 1 (egr1), a transcription factor (TF) that has been reported to control eye development and function, on retinal differentiation in zebrafish. Specifically, cellular changes in the Egr1-knockdown retinas were characterized by immunohistochemistry at 72 and 120 hours post-fertilization (hpf). The results indicate that Egr1 knockdown specifically suppressed the differentiation of subtypes of amacrine cells (ACs) and horizontal cells (HCs), including Parvalbumin- and GABA-positive ACs as well as Islet1-positive HCs. In addition, the knockdown induced a general delay of development of the other retinal cell types. These differentiation problems, particularly the ones with the ACs and HCs, also compromised the integrity of the inner and outer plexiform layers. In the Egr1-knockdown retinas, the expression of ptf1a, a TF that controls the specification of ACs and HCs, was prolonged and found in ectopic locations in the retina up to 72 hpf. Then, it became restricted to the proliferative marginal zone as in the control retinas at 120 hpf. This abnormal and prolonged expression of ptf1a during retinogenesis might affect the differentiation of ACs and HCs in the Egr1-knockdown retinas. | Immunohistochemistry | 23405257
 |
A Bmp reporter with ultrasensitive characteristics reveals that high Bmp signaling is not required for cortical hem fate.
Doan, LT; Javier, AL; Furr, NM; Nguyen, KL; Cho, KW; Monuki, ES
PloS one
7
e44009
2011
Afficher le résumé
Insights into Bone morphogenetic protein (Bmp) functions during forebrain development have been limited by a lack of Bmp signaling readouts. Here we used a novel Bmp signaling reporter ("BRE-gal" mice) to study Bmp signaling in the dorsal telencephalon. At early stages, BRE-gal expression was restricted to the dorsal telencephalic midline. At later stages, strong BRE-gal expression occurred in neurons of the marginal zone and dentate gyrus. Comparisons to nuclear phospho-Smad1/5/8 (pSmad) and Msx1 indicated that BRE-gal expression occurred exclusively in neural cells with high-level Bmp signaling. BRE-gal responsiveness to Bmps was confirmed in reporter-negative cortical cells cultured with Bmp4, and both in vivo and in vitro, BRE-gal expression was switch-like, or ultrasensitive. In the early dorsal telencephalon, BRE-gal expression negatively correlated with the cortical selector gene Lhx2, indicating a BRE-gal expression border that coincides with the cortex-hem boundary. However, in Lhx2 null chimeras, neither BRE-gal nor nuclear pSmad increases were observed in ectopic hem cells. These findings establish BRE-gal as an ultrasensitive reporter of Bmp signaling in the dorsal telencephalon, imply that hem fate can be specified at different Bmp signaling intensities, and suggest that Lhx2 primarily regulates the responses to--rather than the intensity of--Bmp signaling in dorsal telencephalic cells. | | 22984456
 |
The tumor suppressor gene Trp53 protects the mouse lens against posterior subcapsular cataracts and the BMP receptor Acvr1 acts as a tumor suppressor in the lens.
Wiley, LA; Rajagopal, R; Dattilo, LK; Beebe, DC
Disease models & mechanisms
4
484-95
2010
Afficher le résumé
We previously found that lenses lacking the Acvr1 gene, which encodes a bone morphogenetic protein (BMP) receptor, had abnormal proliferation and cell death in epithelial and cortical fiber cells. We tested whether the tumor suppressor protein p53 (encoded by Trp53) affected this phenotype. Acvr1 conditional knockout (Acvr1(CKO)) mouse fiber cells had increased numbers of nuclei that stained for p53 phosphorylated on serine 15, an indicator of p53 stabilization and activation. Deletion of Trp53 rescued the Acvr1(CKO) cell death phenotype in embryos and reduced Acvr1-dependent apoptosis in postnatal lenses. However, deletion of Trp53 alone increased the number of fiber cells that failed to withdraw from the cell cycle. Trp53(CKO) and Acvr1;Trp53(DCKO) (double conditional knockout), but not Acvr1(CKO), lenses developed abnormal collections of cells at the posterior of the lens that resembled posterior subcapsular cataracts. Cells from human posterior subcapsular cataracts had morphological and molecular characteristics similar to the cells at the posterior of mouse lenses lacking Trp53. In Trp53(CKO) lenses, cells in the posterior plaques did not proliferate but, in Acvr1;Trp53(DCKO) lenses, many cells in the posterior plaques continued to proliferate, eventually forming vascularized tumor-like masses at the posterior of the lens. We conclude that p53 protects the lens against posterior subcapsular cataract formation by suppressing the proliferation of fiber cells and promoting the death of any fiber cells that enter the cell cycle. Acvr1 acts as a tumor suppressor in the lens. Enhancing p53 function in the lens could contribute to the prevention of steroid- and radiation-induced posterior subcapsular cataracts. | | 21504908
 |
Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis.
Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, Feral C, Papayannopoulou T, Lowy A, Blair SL, Cheresh D, Ginsberg M, Varner JA
Cancer Res
70
3042-51. Epub 2010 Apr 13.
2009
Afficher le résumé
Recent studies have shown that lymphangiogenesis or the growth of lymphatic vessels at the periphery of tumors promotes tumor metastasis to lymph nodes. We show here that the fibronectin-binding integrin alpha4beta1 and its ligand fibronectin are novel functional markers of proliferative lymphatic endothelium. Tumors and lymphangiogenic growth factors, such as vascular endothelial growth factor-C (VEGF-C) and VEGF-A, induce lymphatic vessel expression of integrin alpha4beta1. Integrin alpha4beta1 then promotes growth factor and tumor-induced lymphangiogenesis, as genetic loss of integrin alpha4beta1 expression in Tie2Cre+ alpha4(loxp/loxp) mice or genetic loss of alpha4 signaling in alpha4Y991A knock-in mice blocks growth factor and tumor-induced lymphangiogenesis, as well as tumor metastasis to lymph nodes. In addition, antagonists of integrin alpha4beta1 suppress lymphangiogenesis and tumor metastasis. Our studies show that integrin alpha4beta1 and the signals it transduces regulate the adhesion, migration, invasion, and survival of proliferating lymphatic endothelial cells. As suppression of alpha4beta1 expression, signal transduction, or function in tumor lymphatic endothelium not only inhibits tumor lymphangiogenesis but also prevents metastatic disease, these results show that integrin alpha4beta1-mediated tumor lymphangiogenesis promotes metastasis and is a useful target for the suppression of metastatic disease. Article en texte intégral | | 20388801
 |
Activation of ERK by spontaneous seizures in neural progenitors of the dentate gyrus in a mouse model of epilepsy.
Yi Li,Zechun Peng,Bo Xiao,Carolyn R Houser
Experimental neurology
224
2009
Afficher le résumé
Cellular changes that are associated with spontaneous seizures in temporal lobe epilepsy are not well understood but could influence ongoing epilepsy-related processes. In order to identify cell signaling events that could occur at the time of spontaneous seizures, the localization of phosphorylated extracellular signal-regulated kinase (pERK) was studied in a pilocarpine mouse model of epilepsy at very short intervals (1.5-2.5 min) after detection of a spontaneous seizure. Within the hippocampal formation, immunolabeling of pERK was evident in a subpopulation of cells in the subgranular zone (SGZ) of the dentate gyrus at these short intervals. Many of these cells had a long vertical process and resembled radial glia, while others had short processes and were oriented horizontally. Labeling with a series of developmental markers demonstrated that virtually all pERK-labeled cells were neural progenitor cells (NPCs). A high percentage ( approximately 80%) of the pERK-labeled cells was labeled with either glial fibrillary acidic protein or brain lipid binding protein, indicating that these cells were radial glia-like NPCs. A smaller percentage of labeled cells expressed NeuroD, suggesting that they were later-developing NPCs that were assuming a neuronal identity. Early expression of pERK was not detected in immature neurons. Double labeling with proliferation markers demonstrated that approximately 30% of pERK-labeled NPCs expressed Mcm2, indicating that they were actively proliferating. Furthermore, virtually all radial glia-like NPCs that were in the proliferative cycle expressed pERK. These findings suggest that spontaneous seizures and associated ERK activation could contribute to the proliferation of radial glia-like NPCs in this epilepsy model. Article en texte intégral | | 20226181
 |
The tumor suppressor merlin is required for cell cycle exit, terminal differentiation, and cell polarity in the developing murine lens.
Luke A Wiley,Lisa K Dattilo,Kai B Kang,Marco Giovannini,David C Beebe
Investigative ophthalmology & visual science
51
2009
Afficher le résumé
PURPOSE. Neurofibromatosis type 2 (NF2) is an autosomal-dominant CNS tumor syndrome that affects 1:25,000 children and young adults. More than 50% of NF2 patients also develop posterior subcapsular cataracts (PSCs). The authors deleted Nf2 from the lens to determine its role in fiber cell differentiation. METHODS. Nf2 was conditionally deleted from murine lenses using the LeCre transgene. Standard histology and immunohistochemical and immunofluorescent methods were used to analyze lens morphology and markers of cell cycle progression, differentiation, and cell junctions in wild-type and knockout lenses from embryonic day 10.5 through postnatal day 3. RESULTS. Fiber cells lacking Nf2 did not fully exit the cell cycle and continued to express epithelial cell markers, such as FoxE3 and E-cadherin, despite expressing the fiber cell marker Prox1. Many fiber cells lost their elongated morphology. Markers of apical-basal polarity, such as ZO-1, were mislocalized along the lateral and basal membranes of fiber cells. The lens vesicle failed to separate from the surface ectoderm, and prospective lens and corneal epithelial cells formed a multilayered mass of cells at the surface of the eye. Herniation of this membrane caused the fiber mass to erupt through the cornea. CONCLUSIONS. Nf2 is required for complete fiber cell terminal differentiation, maintenance of cell polarity, and separation of lens vesicle from corneal epithelium. Defects identified in fiber cell differentiation may explain the formation of PSCs in patients with NF2. The lens provides an assay system to identify pathways critical for fiber cell differentiation and to test therapies for the tumors that occur in patients with NF2. Article en texte intégral | | 20181838
 |
Production of monoclonal antibodies against Prox1.
Chen, Xiaoren, et al.
Hybridoma (Larchmt), 25: 27-33 (2006)
2005
Afficher le résumé
Prox1 is a divergent homeodomain protein important for the development of the lens, retina, liver, pancreas, and lymphatic vasculature. Prox1 expression is highly upregulated in transformed hepatocytes and has been used as a marker to distinguish lymphatic from blood vasculature. We produced recombinant human Prox1 (amino acids 547-737) fused to glutathione S-transferase (GST) and used it to create two hybridomas, 5G10 and 4G10. Both of these hybridomas produced monoclonal antibodies able to detect Prox1 by immunofluorescence in lenses from diverse terrestrial vertebrates, including humans, rats, chickens, and lizards, although 5G10 was generally more sensitive in this application. Further, 4G10 was able to robustly detect endogenous and recombinant Prox1 in both cell and tissue extracts by Western blotting, while 5G10 was notably less sensitive for this purpose. These monoclonal antibodies will be useful for diverse studies on the role of Prox1 in both normal development and disease processes in terrestrial vertebrates. | | 16475879
 |