Spatial mapping of juxtacrine axo-glial interactions identifies novel molecules in peripheral myelination.
Poitelon, Y; Bogni, S; Matafora, V; Della-Flora Nunes, G; Hurley, E; Ghidinelli, M; Katzenellenbogen, BS; Taveggia, C; Silvestri, N; Bachi, A; Sannino, A; Wrabetz, L; Feltri, ML
Nature communications
6
8303
2015
Afficher le résumé
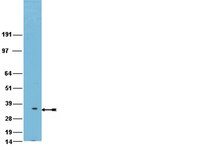
Cell-cell interactions promote juxtacrine signals in specific subcellular domains, which are difficult to capture in the complexity of the nervous system. For example, contact between axons and Schwann cells triggers signals required for radial sorting and myelination. Failure in this interaction causes dysmyelination and axonal degeneration. Despite its importance, few molecules at the axo-glial surface are known. To identify novel molecules in axo-glial interactions, we modified the 'pseudopodia' sub-fractionation system and isolated the projections that glia extend when they receive juxtacrine signals from axons. By proteomics we identified the signalling networks present at the glial-leading edge, and novel proteins, including members of the Prohibitin family. Glial-specific deletion of Prohibitin-2 in mice impairs axo-glial interactions and myelination. We thus validate a novel method to model morphogenesis and juxtacrine signalling, provide insights into the molecular organization of the axo-glial contact, and identify a novel class of molecules in myelination. | Immunohistochemistry | 26383514
 |
Involvement of de novo synthesized palmitate and mitochondrial EGFR in EGF induced mitochondrial fusion of cancer cells.
Bollu, LR; Ren, J; Blessing, AM; Katreddy, RR; Gao, G; Xu, L; Wang, J; Su, F; Weihua, Z
Cell cycle (Georgetown, Tex.)
13
2415-30
2014
Afficher le résumé
Increased expressions of fatty acid synthase (FASN) and epidermal growth factor receptor (EGFR) are common in cancer cells. De novo synthesis of palmitate by FASN is critical for the survival of cancer cells via mechanisms independent of its role as an energy substrate. Besides the plasma membrane and the nucleus, EGFR can also localize at the mitochondria; however, signals that can activate mitochondrial EGFR (mtEGFR) and the functions of mtEGFR of cancer cells remain unknown. The present study characterizes mtEGFR in the mitochondria of cancer cells (prostate and breast) and reveals that mtEGFR can promote mitochondrial fusion through increasing the protein levels of fusion proteins PHB2 and OPA1. Activation of plasma membranous EGFR (pmEGFR) stimulates the de novo synthesis of palmitate through activation of FASN and ATP-citrate lyase (ACLy). In vitro kinase assay with isolated mitochondria shows that palmitate can activate mtEGFR. Inhibition of FASN blocks the mtEGFR phosphorylation and palmitoylation induced by EGF. Mutational studies show that the cysteine 797 is important for mtEGFR activation and palmitoylation. Inhibition of FASN can block EGF induced mitochondrial fusion and increased the sensitivity of prostate cancer cells to EGFR tyrosine kinase inhibitor. In conclusion, these results suggest that mtEGFR can be activated by pmEGFR through de novo synthesized palmitate to promote mitochondrial fusion and survival of cancer cells. This mechanism may serve as a novel target to improve EGFR-based cancer therapy. | | 25483192
 |
PHB2 protects sister-chromatid cohesion in mitosis.
Takata, Hideaki, et al.
Curr. Biol., 17: 1356-61 (2007)
2007
Afficher le résumé
Cohesion between sister chromatids is essential for proper chromosome segregation in mitosis. In vertebrate mitotic cells, most cohesin is removed from the chromosome arms [1-4], but centromeric cohesin is protected by shugoshin until the onset of anaphase [5]. However, the mechanism of this protection of centromeric cohesion is not well understood. Here, we demonstrate that prohibitin 2 (PHB2) is involved in the regulation of sister-chromatid cohesion during mitosis in HeLa cells. PHB2 is an evolutionarily conserved protein in eukaryotes and has multiple functions, such as transcriptional regulation and cell viability and development [6-8]. However, its functions in mitosis have not yet been determined. We show that depletion of PHB2 by RNA interference (RNAi) causes premature sister-chromatid separation and defects in chromosome congression accompanied by mitotic arrest by spindle-checkpoint activation. In the absence of PHB2, cohesin is dissociated from centromeres during early mitosis, although the centromeric localization of shugoshin is preserved. Thus, our findings suggest that, in addition to the shugoshin, PHB2 is also required to protect the centromeric cohesion from phosphorylation by Plk1 during early mitosis and that its function is essential for proper mitotic progression. | | 17656096
 |