Cyp1b1 mediates periostin regulation of trabecular meshwork development by suppression of oxidative stress.
Zhao, Y; Wang, S; Sorenson, CM; Teixeira, L; Dubielzig, RR; Peters, DM; Conway, SJ; Jefcoate, CR; Sheibani, N
Molecular and cellular biology
33
4225-40
2013
Afficher le résumé
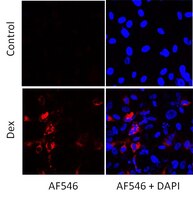
Mutation in CYP1B1 has been reported for patients with congenital glaucoma. However, the underlying mechanisms remain unknown. Here we show increased diurnal intraocular pressure (IOP) in Cyp1b1-deficient (Cyp1b1(-/-)) mice. Cyp1b1(-/-) mice presented ultrastructural irregular collagen distribution in their trabecular meshwork (TM) tissue along with increased oxidative stress and decreased levels of periostin (Postn). Increased levels of oxidative stress and decreased levels of Postn were also detected in human glaucomatous TM tissues. Furthermore, Postn-deficient mice exhibited TM tissue ultrastructural abnormalities similar to those of Cyp1b1(-/-) mice. Administration of the antioxidant N-acetylcysteine (NAC) restored structural abnormality of TM tissue in Cyp1b1(-/-) mice. In addition, TM cells prepared from Cyp1b1(-/-) mice exhibited increased oxidative stress, altered adhesion, and decreased levels of Postn. These aberrant cellular responses were reversed in the presence of NAC or by restoration of Cyp1b1 expression. Cyp1b1 knockdown or inhibition of CYP1B1 activity in Cyp1b1(+/+) TM cells resulted in a Cyp1b1(-/-) phenotype. Thus, metabolic activity of CYP1B1 contributes to oxidative homeostasis and ultrastructural organization and function of TM tissue through modulation of Postn expression. | Dot Blot | Mouse | 23979599
 |
Characterization of monoclonal antibodies against the glaucoma-associated protein myocilin.
Ezzat, MK; Howell, KG; Bahler, CK; Beito, TG; Loewen, N; Poeschla, EM; Fautsch, MP
Experimental eye research
87
376-84
2008
Afficher le résumé
Although the glaucoma-associated protein myocilin has been the focus of intensive research, its biological function is still unknown. One of the limiting factors has been the lack of well-characterized antibodies, particularly monoclonal antibodies. We describe the development of six monoclonal antibodies specific to myocilin and characterize their suitability in Western blot and immunohistochemical applications. Three of the six monoclonal antibodies recognize the N-terminus of myocilin (amino acids 33-214), two antibodies recognize the middle third of the protein (amino acids 215-368), and one antibody recognizes the C-terminus (amino acids 369-504). Isotyping revealed that all antibodies are of the IgG1 kappa class except one, which is IgG2b kappa. Purified myocilin monoclonal antibodies were able to recognize myocilin in human aqueous humor separated on denatured/reduced and native gels, and human trabecular meshwork lysate by Western blot. Myocilin was also detected by immunohistochemistry in trabecular meshwork, ciliary body, iris, cornea, sclera, choroid, and retinal pigment epithelial cells. | | | 18674535
 |