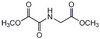
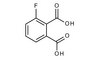
Opposing effects of collagen I and vitronectin on fibronectin fibril structure and function.
Gildner, CD; Roy, DC; Farrar, CS; Hocking, DC
Matrix biology : journal of the International Society for Matrix Biology
34
33-45
2014
Afficher le résumé
Extracellular matrix fibronectin fibrils serve as passive structural supports for the organization of cells into tissues, yet can also actively stimulate a variety of cell and tissue functions, including cell proliferation. Factors that control and coordinate the functional activities of fibronectin fibrils are not known. Here, we compared effects of cell adhesion to vitronectin versus type I collagen on the assembly of and response to, extracellular matrix fibronectin fibrils. The amount of insoluble fibronectin matrix fibrils assembled by fibronectin-null mouse embryonic fibroblasts adherent to collagen- or vitronectin-coated substrates was not significantly different 20 h after fibronectin addition. However, the fibronectin matrix produced by vitronectin-adherent cells was ~10-fold less effective at enhancing cell proliferation than that of collagen-adherent cells. Increasing insoluble fibronectin levels with the fibronectin fragment, anastellin did not increase cell proliferation. Rather, native fibronectin fibrils polymerized by collagen- and vitronectin-adherent cells exhibited conformational differences in the growth-promoting, III-1 region of fibronectin, with collagen-adherent cells producing fibronectin fibrils in a more extended conformation. Fibronectin matrix assembly on either substrate was mediated by α5β1 integrins. However, on vitronectin-adherent cells, α5β1 integrins functioned in a lower activation state, characterized by reduced 9EG7 binding and decreased talin association. The inhibitory effect of vitronectin on fibronectin-mediated cell proliferation was localized to the cell-binding domain, but was not a general property of αvβ3 integrin-binding substrates. These data suggest that adhesion to vitronectin allows for the uncoupling of fibronectin fibril formation from downstream signaling events by reducing α5β1 integrin activation and fibronectin fibril extension. | | 24509439
 |
Recombinant fibronectin matrix mimetics specify integrin adhesion and extracellular matrix assembly.
Roy, DC; Hocking, DC
Tissue engineering. Part A
19
558-70
2013
Afficher le résumé
Tissue engineering seeks to create functional tissues and organs by integrating natural or synthetic scaffolds with bioactive factors and cells. Creating biologically active scaffolds that support key aspects of tissue regeneration, including the re-establishment of a functional extracellular matrix (ECM), is a challenge currently facing this field. During tissue repair, fibronectin is converted from an inactive soluble form into biologically active ECM fibrils through a cell-dependent process. ECM fibronectin promotes cell processes critical to tissue regeneration and regulates the deposition and organization of other ECM proteins. We previously developed biomimetics of ECM fibronectin by directly coupling the heparin-binding fragment of the first type III repeat of fibronectin (FNIII1H) to the integrin-binding repeats (FNIII8-10). As adhesive substrates, fibronectin matrix mimetics promote cell growth, migration, and contractility through a FNIII1H-dependent mechanism. Here, we analyzed fibronectin matrix mimetic variants designed to include all or part of the integrin-binding domain for their ability to support new ECM assembly. We found that specific modifications of the integrin-binding domain produced adhesive substrates that selectively engage different integrin receptors to, in turn, regulate the amount of fibronectin and collagen deposited into the ECM. The ability of fibronectin matrix mimetics to direct cell-substrate interactions and regulate ECM assembly makes them promising candidates for use as bioactive surfaces, where precise control over integrin-binding specificity and ECM deposition are required. | | 23020251
 |
Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension.
Coyer, SR; Singh, A; Dumbauld, DW; Calderwood, DA; Craig, SW; Delamarche, E; García, AJ
Journal of cell science
125
5110-23
2011
Afficher le résumé
Integrin-based focal adhesions (FA) transmit anchorage and traction forces between the cell and the extracellular matrix (ECM). To gain further insight into the physical parameters of the ECM that control FA assembly and force transduction in non-migrating cells, we used fibronectin (FN) nanopatterning within a cell adhesion-resistant background to establish the threshold area of ECM ligand required for stable FA assembly and force transduction. Integrin-FN clustering and adhesive force were strongly modulated by the geometry of the nanoscale adhesive area. Individual nanoisland area, not the number of nanoislands or total adhesive area, controlled integrin-FN clustering and adhesion strength. Importantly, below an area threshold (0.11 µm(2)), very few integrin-FN clusters and negligible adhesive forces were generated. We then asked whether this adhesive area threshold could be modulated by intracellular pathways known to influence either adhesive force, cytoskeletal tension, or the structural link between the two. Expression of talin- or vinculin-head domains that increase integrin activation or clustering overcame this nanolimit for stable integrin-FN clustering and increased adhesive force. Inhibition of myosin contractility in cells expressing a vinculin mutant that enhances cytoskeleton-integrin coupling also restored integrin-FN clustering below the nanolimit. We conclude that the minimum area of integrin-FN clusters required for stable assembly of nanoscale FA and adhesive force transduction is not a constant; rather it has a dynamic threshold that results from an equilibrium between pathways controlling adhesive force, cytoskeletal tension, and the structural linkage that transmits these forces, allowing the balance to be tipped by factors that regulate these mechanical parameters. | | 22899715
 |
Morphological and histological evaluations of 3D-layered blood vessel constructs prepared by hierarchical cell manipulation.
Michiya Matsusaki,Koji Kadowaki,Eijiro Adachi,Takeshi Sakura,Utako Yokoyama,Yoshihiro Ishikawa,Mitsuru Akashi
Journal of biomaterials science. Polymer edition
23
2011
Afficher le résumé
Three-dimensional (3D)-layered blood vessel constructs consisting of human umbilical artery smooth muscle cells (SMCs) and human umbilical vascular endothelial cells (ECs) were fabricated by hierarchical cell manipulation, and their basic morphology, histology and blood compatibility were evaluated in relation to the EC layers. For the hierarchical cell manipulation, fibronectin-gelatin (FN-G) nanofilms were prepared on the surface of SMC layers to provide a cell adhesive nano-scaffold for the second layer of cells. The layer number of blood vessel constructs was easily controllable from 2 to 7 layers, and the histological evaluation, scanning electron microscope (SEM) and transmission electron microscope (TEM) observations indicated a hierarchical blood vessel analogous morphology. The immunefluorescence staining revealed homogeneous and dense tight-junction of the uppermost EC layer. Furthermore, the nano-meshwork morphology of the FN-G films like a native extracellular matrix was observed inside the blood vessel constructs by SEM. Moreover, a close association between actin microfilaments and the nano-meshworks was observed on the SMC surface by TEM. The blood compatibility of the blood vessel constructs, 4-layered SMC/1-layered EC (4L-SMC/1L-EC), was clearly confirmed by inhibition of platelet adhesion, whereas the blood vessel constructs without EC layers (4L-SMC) showed high adhesion and activation of the platelet. The 3D-blood vessel constructs prepared by hierarchical cell manipulation technique will be valuable as a blood vessel model in the tissue engineering or pharmaceutical fields. | | 21176392
 |
Serum response factor is required for cell contact maintenance but dispensable for proliferation in visceral yolk sac endothelium.
Holtz, ML; Misra, RP
BMC developmental biology
11
18
2010
Afficher le résumé
Endothelial-specific knockout of the transcription factor serum response factor (SRF) results in embryonic lethality by mid-gestation. The associated phenotype exhibits vascular failure in embryos as well as visceral yolk sac (VYS) tissues. Previous data suggest that this vascular failure is caused by alterations in cell-cell and cell-matrix contacts. In the current study, we sought to more carefully address the role of SRF in endothelial function and cell contact interactions in VYS tissues.Tie2-Cre recombinase-mediated knockout of SRF expression resulted in loss of detectable SRF from VYS mesoderm by E12.5. This loss was accompanied by decreased expression of smooth muscle alpha-actin as well as vascular endothelial cadherin and claudin 5, endothelial-specific components of adherens and tight junctions, respectively. Focal adhesion (FA) integrins alpha5 and beta1 were largely unchanged in contrast to loss of the FA-associated molecule vinculin. The integrin binding partner fibronectin-1 was also profoundly decreased in the extracellular matrix, indicating another aspect of impaired adhesive function and integrin signaling. Additionally, cells in SRF-null VYS mesoderm failed to reduce proliferation, suggesting not only that integrin-mediated contact inhibition is impaired but also that SRF protein is not required for proliferation in these cells.Our data support a model in which SRF is critical in maintaining functional cell-cell and cell-matrix adhesion in endothelial cells. Furthermore, we provide evidence that supports a model in which loss of SRF protein results in a sustained proliferation defect due in part to failed integrin signaling. Article en texte intégral | | 21401944
 |
Pressure load: the main factor for altered gene expression in right ventricular hypertrophy in chronic hypoxic rats.
Baandrup, JD; Markvardsen, LH; Peters, CD; Schou, UK; Jensen, JL; Magnusson, NE; Ørntoft, TF; Kruhøffer, M; Simonsen, U
PloS one
6
e15859
2010
Afficher le résumé
The present study investigated whether changes in gene expression in the right ventricle following pulmonary hypertension can be attributed to hypoxia or pressure loading.To distinguish hypoxia from pressure-induced alterations, a group of rats underwent banding of the pulmonary trunk (PTB), sham operation, or the rats were exposed to normoxia or chronic, hypobaric hypoxia. Pressure measurements were performed and the right ventricle was analyzed by Affymetrix GeneChip, and selected genes were confirmed by quantitative PCR and immunoblotting. Right ventricular systolic blood pressure and right ventricle to body weight ratio were elevated in the PTB and the hypoxic rats. Expression of the same 172 genes was altered in the chronic hypoxic and PTB rats. Thus, gene expression of enzymes participating in fatty acid oxidation and the glycerol channel were downregulated. mRNA expression of aquaporin 7 was downregulated, but this was not the case for the protein expression. In contrast, monoamine oxidase A and tissue transglutaminase were upregulated both at gene and protein levels. 11 genes (e.g. insulin-like growth factor binding protein) were upregulated in the PTB experiment and downregulated in the hypoxic experiment, and 3 genes (e.g. c-kit tyrosine kinase) were downregulated in the PTB and upregulated in the hypoxic experiment.Pressure load of the right ventricle induces a marked shift in the gene expression, which in case of the metabolic genes appears compensated at the protein level, while both expression of genes and proteins of importance for myocardial function and remodelling are altered by the increased pressure load of the right ventricle. These findings imply that treatment of pulmonary hypertension should also aim at reducing right ventricular pressure. Article en texte intégral | | 21246034
 |
CCN2 (Connective Tissue Growth Factor) is essential for extracellular matrix production and integrin signaling in chondrocytes.
Nishida, T; Kawaki, H; Baxter, RM; Deyoung, RA; Takigawa, M; Lyons, KM
Journal of cell communication and signaling
1
45-58
2007
Afficher le résumé
The matricellular protein CCN2 (Connective Tissue Growth Factor; CTGF) is an essential mediator of ECM composition, as revealed through analysis of Ccn2 deficient mice. These die at birth due to complications arising from impaired endochondral ossification. However, the mechanism(s) by which CCN2 mediates its effects in cartilage are unclear. We investigated these mechanisms using Ccn2 ( -/- ) chondrocytes. Expression of type II collagen and aggrecan were decreased in Ccn2 (-/-) chondrocytes, confirming a defect in ECM production. Ccn2 ( -/- ) chondrocytes also exhibited impaired DNA synthesis and reduced adhesion to fibronectin. This latter defect is associated with decreased expression of alpha5 integrin. Moreover, CCN2 can bind to integrin alpha5beta1 in chondrocytes and can stimulate increased expression of integrin alpha5. Consistent with an essential role for CCN2 as a ligand for integrins, immunofluorescence and Western blot analysis revealed that levels of focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK)1/2 phosphorylation were reduced in Ccn2 ( -/- ) chondrocytes. These findings argue that CCN2 exerts major effects in chondrocytes through its ability to (1) regulate ECM production and integrin alpha5 expression, (2) engage integrins and (3) activate integrin-mediated signaling pathways. Article en texte intégral | Immunoprecipitation | 18481209
 |
The role of integrin alpha5beta1 in the regulation of corneal neovascularization.
Philipp S Muether, Susanne Dell, Norbert Kociok, Grit Zahn, Roland Stragies, Doerte Vossmeyer, Antonia M Joussen
Experimental eye research
85
356-65
2007
Afficher le résumé
Integrins are transmembrane receptor proteins critical for growth and stabilization of vessels, but the mechanisms by which integrin activities are involved in neoangiogenesis of the eye remain unclear. Specific inhibitors to fibronectin receptor integrin alpha(5)beta(1) impeded pathological neovascularization in vivo. Our objective was to determine whether alpha(5)beta(1) plays a role in ocular angiogenesis, and whether a novel alpha(5)beta(1)-inhibiting small molecule is able to reduce angiogenesis in a model of inflammatory corneal neovascularization. Corneal neovascularization was induced in C57Bl/6 mice by NaOH-application and debridement of the limbal epithelium. Mice were randomized into six groups receiving either no treatment, or intraperitoneal osmotic pumps delivering three different doses of integrin antagonist or control substance on day 10 after scraping. In order to quantify the neovascular response, flatmounts were stained with FITC-CD31. Integrin alpha(5) expression was determined by immunohistochemistry and quantified by semiquantitative western blot analysis. Influence of integrin antagonist treatment on the mRNA expression of VEGF, bFGF and integrin alpha(5) was quantified by real-time RT-PCR. Vascularized corneas demonstrated a strong up-regulation of integrin alpha(5) within affected areas. Animals treated systemically with alpha(5)beta(1)-inhibiting small molecule showed a significant inhibition and regression of corneal neovascularization. PCR analysis evinced a significant up-regulation of VEGF and integrin alpha(5) mRNA levels in injured animals compared to controls, and a significant reduction of integrin alpha(5) mRNA in substance-treated animals compared to control substance, but no significant differences of bFGF levels in all groups. Western blot analysis of integrin alpha(5)beta(1) protein expression showed a trend towards up-regulation in injured animals, both control substance-treated and those treated with the alpha(5)beta(1)-inhibiting small molecule. Systemic delivery of an alpha(5)beta(1)-inhibiting small molecule inhibits and regresses corneal neovascularization induced by mechanical-alkali burn corneal injury. These results suggest an essential role for the integrin alpha(5)beta(1) in pathological neovascular processes of the cornea. Integrin alpha(5)beta(1) inhibitors could become a new approach for treatment of neovascularization in the eye. | | 17659277
 |
Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports.
Timothy A Petrie, Jeffrey R Capadona, Catherine D Reyes, Andrés J García
Biomaterials
27
5459-70
2005
Afficher le résumé
Biomimetic strategies focusing on presenting short bioadhesive oligopeptides, including the arginine-glycine-aspartic acid (RGD) motif present in numerous adhesive proteins, on a non-fouling support have emerged as promising approaches to improve cellular activities and healing responses. Nevertheless, these bio-inspired strategies are limited by low activity of the oligopeptides compared to the native ligand due to the absence of complementary or modulatory domains. In the present analysis, we generated well-defined biointerfaces presenting RGD-based ligands of increasing complexity to directly compare their biological activities in terms of cell adhesion strength, integrin binding and signaling. Mixed self-assembled monolayers of alkanethiols on gold were optimized to engineer robust supports that present anchoring groups for ligand tethering within a non-fouling, protein adsorption-resistant background. Controlled bioadhesive interfaces were generated by tethering adhesive ligands via standard peptide chemistry. On a molar basis, biointerfaces functionalized with the FNIII7-10 recombinant fragment presenting the RGD and PHSRN adhesive motifs in the correct structural context exhibited significantly higher adhesion strength, FAK activation, and cell proliferation rate than supports presenting RGD ligand or RGD-PHSRN, an oligopeptide presenting these two sites separated by a polyglycine linker. Moreover, FNIII7-10-functionalized surfaces displayed specificity for alpha5beta1 integrin, while cell adhesion to supports presenting RGD or RGD-PHSRN was primarily mediated by alphavbeta3 integrin. These results are significant to the rational engineering of bioactive materials that convey integrin binding specificity for directed cellular and tissue responses in biomedical and biotechnological applications. | | 16846640
 |
Spatial expressions of fibronectin and integrins by human and rodent dermal fibroblasts.
M Yasuda, Y Miyachi, O Ishikawa, K Takahashi
The British journal of dermatology
155
522-31
2005
Afficher le résumé
BACKGROUND: Human skin shows various morphological characteristics, depending on the body site. As these distinct phenotypes have been explained on the basis of the variance in epidermal keratinocytes and the presence of skin appendages, the spatial distinction of the dermal components has not been fully elucidated. OBJECTIVES: To identify and characterize the profiles of mRNAs that are abundantly or specifically expressed by fibroblasts derived from trunk skin, but not from palmoplantar skin or oral mucosa. METHODS: In order to identify the distinct mRNA expression by trunk skin fibroblasts, a subtraction cDNA screening was performed first, followed by Northern blotting, Western blotting and immunohistochemistry for cultured human and rat dermal fibroblasts and those skin tissues. Finally, whole mount in situ hybridization (WISH) was performed to examine the differences in the expression of the corresponding gene during the developmental stage of mouse embryos. RESULTS: We identified three cDNA clones encoding fibronectin (FN), pregnancy-specific beta1-glycoprotein 5 and beta-actin, respectively, whose mRNAs were abundantly or specifically expressed by trunk skin fibroblasts. FN and some integrins were further confirmed to be expressed more selectively in human and rat trunk skin fibroblasts, both in terms of the RNA and the protein levels, compared with the fibroblasts derived from plamoplantar skin and oral mucosa. WISH demonstrated that FN was localized around the hair follicles of mouse embryos. CONCLUSIONS: FN, one of most potent extracellular matrix molecules, was demonstrated to be spatially transcribed depending on the body sites. The distinct expression of FN was suggestive of the essential commitment in the process of cutaneous development and morphogenesis of appendages originated from hair germ. The paucity of FN in palmoplantar skin and oral mucosa might explain the characteristics of these skin phenotypes. | | 16911276
 |