386032 Sigma-AldrichAnti-Hsp70 Mouse mAb (C92F3A-5)
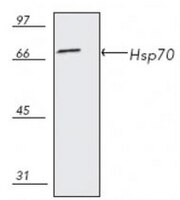
Anti-Hsp70, mouse monoclonal, clone C92F3A-5, recognizes the ~70 kDa Hsp70. Does not cross-react with Hsc70. It is validated for use in ELISA, FC, WB, ICC, IP & IHC (frozen and paraffin sections).
More>> Anti-Hsp70, mouse monoclonal, clone C92F3A-5, recognizes the ~70 kDa Hsp70. Does not cross-react with Hsc70. It is validated for use in ELISA, FC, WB, ICC, IP & IHC (frozen and paraffin sections). Less<<Synonymes: Anti-Heat Shock Protein 70
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| Species Reactivity | Host | Antibody Type |
|---|---|---|
| A Broad Range Of Species | M | Monoclonal Antibody |
Products
| Référence | Conditionnement | Qté | |
|---|---|---|---|
| 386032-50UG | Ampoule plast. | 50 μg |
| Product Information | |
|---|---|
| Form | Liquid |
| Formulation | In PBS, 50% glycerol, pH 7.2. |
| Positive control | L929 cells, Human colon cancer tissue |
| Preservative | ≤0.1% sodium azide |
| Quality Level | MQ100 |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 386032-50UG | 04055977189889 |
Documentation
Anti-Hsp70 Mouse mAb (C92F3A-5) FDS
| Titre |
|---|
Anti-Hsp70 Mouse mAb (C92F3A-5) Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 386032 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Hang, H., and Fox, M.H. 1995. Cytometry 19, 119. Kilgore, J.L., et al. 1994. J. Appl. Physiol. 76, 589. Heufelder, A.E., et al. 1992. J. Clin. Endocrinol. Metab. 74, 724. Gower, D.J., et al. 1989. J. Neurosurg. 70, 605. Milarski, K., et al. 1989.J. Cell Biol. 108, 413. Vass, K., et al. 1988. Acta Neuropathologica 77, 128. Welch, W.J., and Suhan, J.P. 1986. J. Cell Biol. 103, 2035. |