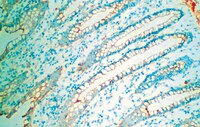
Macromolecular organization and fine structure of the human basilar membrane - RELEVANCE for cochlear implantation.
Liu, W; Atturo, F; Aldaya, R; Santi, P; Cureoglu, S; Obwegeser, S; Glueckert, R; Pfaller, K; Schrott-Fischer, A; Rask-Andersen, H
Cell and tissue research
360
245-62
2015
Afficher le résumé
Cochlear micromechanics and frequency tuning depend on the macromolecular organization of the basilar membrane (BM), which is still unclear in man. Novel techniques in cochlear implantation (CI) motivate further analyses of the BM.Normal cochleae from patients undergoing removal of life-threatening petro-clival meningioma and an autopsy specimen from a normal human were used. Laser-confocal microscopy, high resolution scanning (SEM) and transmission electron microscopy (TEM) were carried out in combination. In addition, one human temporal bone was decellularized and investigated by SEM.The human BM consisted in four separate layers: (1) epithelial basement membrane positive for laminin-β2 and collagen IV, (2) BM "proper" composed of radial fibers expressing collagen II and XI, (3) layer of collagen IV and (4) tympanic covering layer (TCL) expressing collagen IV, fibronectin and integrin. BM thickness varied both radially and longitudinally (mean 0.55-1.16 μm). BM was thinnest near the OHC region and laterally.There are several important similarities and differences between the morphology of the BM in humans and animals. Unlike in animals, it does not contain a distinct pars tecta (arcuate) and pectinata. Its width increases and thickness decreases as it travels apically in the cochlea. Findings show that the human BM is thinnest and probably most vibration-sensitive at the outer pillar feet/Deiter cells at the OHCs. The inner pillar and IHCs seem situated on a fairly rigid part of the BM. The gradient design of the BM suggests that its vulnerability increases apical wards when performing hearing preservation CI surgery. | | 25663274
 |
Oncogenic targeting of BRM drives malignancy through C/EBPβ-dependent induction of α5 integrin.
Damiano, L; Stewart, KM; Cohet, N; Mouw, JK; Lakins, JN; Debnath, J; Reisman, D; Nickerson, JA; Imbalzano, AN; Weaver, VM
Oncogene
33
2441-53
2014
Afficher le résumé
Integrin expression and activity are altered in tumors, and aberrant integrin signaling promotes malignancy. However, how integrins become altered in tumors remains poorly understood. We discovered that oncogenic activation of MEK signaling induces cell growth and survival, and promotes the malignant phenotype of mammary epithelial cells (MECs) by increasing α5 integrin expression. We determined that MEK activates c-Myc to reduce the transcription of the SWI/SNF chromatin remodeling enzyme Brahma (BRM). Our studies revealed that reduced BRM expression and/or activity drives the malignant behavior of MECs by epigenetically promoting C/EBPβ expression to directly induce α5 integrin transcription. Consistently, we could show that restoring BRM levels normalized the malignant behavior of transformed MECs in culture and in vivo by preventing C/EBPβ-dependent α5 integrin transcription. Our findings identify a novel mechanism whereby oncogenic signaling promotes malignant transformation by regulating transcription of a key chromatin remodeling molecule that regulates integrin-dependent stromal-epithelial interactions. | | 23770848
 |
Ail protein binds ninth type III fibronectin repeat (9FNIII) within central 120-kDa region of fibronectin to facilitate cell binding by Yersinia pestis.
Tiffany M Tsang,Douglas S Annis,Malte Kronshage,Jesse T Fenno,Lisa D Usselman,Deane F Mosher,Eric S Krukonis
The Journal of biological chemistry
287
2011
Afficher le résumé
The Yersinia pestis adhesin molecule Ail interacts with the extracellular matrix protein fibronectin (Fn) on host cells to facilitate efficient delivery of cytotoxic Yop proteins, a process essential for plague virulence. A number of bacterial pathogens are known to bind to the N-terminal region of Fn, comprising type I Fn (FNI) repeats. Using proteolytically generated Fn fragments and purified recombinant Fn fragments, we demonstrated that Ail binds the centrally located 120-kDa fragment containing type III Fn (FNIII) repeats. A panel of monoclonal antibodies (mAbs) that recognize specific epitopes within the 120-kDa fragment demonstrated that mAb binding to (9)FNIII blocks Ail-mediated bacterial binding to Fn. Epitopes of three mAbs that blocked Ail binding to Fn were mapped to a similar face of (9)FNIII. Antibodies directed against (9)FNIII also inhibited Ail-dependent cell binding activity, thus demonstrating the biological relevance of this Ail binding region on Fn. Bacteria expressing Ail on their surface could also bind a minimal fragment of Fn containing repeats (9-10)FNIII, and this binding was blocked by a mAb specific for (9)FNIII. These data demonstrate that Ail binds to (9)FNIII of Fn and presents Fn to host cells to facilitate cell binding and delivery of Yops (cytotoxins of Y. pestis), a novel interaction, distinct from other bacterial Fn-binding proteins. | | 22447929
 |
Enhanced focal adhesion assembly reflects increased mechanosensation and mechanotransduction at maternal-conceptus interface and uterine wall during ovine pregnancy.
Burghardt, RC; Burghardt, JR; Taylor, JD; Reeder, AT; Nguen, BT; Spencer, TE; Bayless, KJ; Johnson, GA
Reproduction (Cambridge, England)
137
567-82
2009
Afficher le résumé
The integrity of the fetal-maternal interface is critical for proper fetal nourishment during pregnancy. Integrins are important adhesion molecules present at the interface during implantation; however, in vivo evidence for integrin activation and focal adhesion formation at the maternal-conceptus interface is limited. We hypothesized that focal adhesion assembly in uterine luminal epithelium (LE) and conceptus trophectoderm (Tr) results from integrin binding of extracellular matrix (ECM) at this interface to provide increased tensile forces and signaling to coordinate utero-placental development. An ovine model of unilateral pregnancy was used to evaluate mechanotransduction events leading to focal adhesion assembly at the maternal-conceptus interface and within the uterine wall. Animals were hysterectomized on days 40, 80, or 120 of pregnancy, and uteri immunostained for integrins (ITGAV, ITGA4, ITGA5, ITGB1, ITGB3, and ITGB5), ECM proteins (SPP1, LGALS15, fibronectin (FN), and vitronectin (VTN)), cytoskeletal molecules (ACTN and TLN1), and a signal generator (PTK2). Focal adhesion assembly in myometrium and stroma was also studied to provide a frame of reference for mechanical stretch of the uterine wall. Large focal adhesions containing aggregates of ITGAV, ITGA4, ITGA5, ITGB1, ITGB5, ACTN, and PTK2 were detected in interplacentomal uterine LE and Tr of gravid but not non-gravid uterine horns and increased during pregnancy. SPP1 and LGALS15, but not FN or VTN, were present along LE and Tr interfaces in both uterine horns. These data support the idea that focal adhesion assembly at the maternal-conceptus interface reflects adaptation to increasing forces caused by the growing fetus. Cooperative binding of multiple integrins to SPP1 deposited at the maternal-conceptus interface forms an adhesive mosaic to maintain a tight connection between uterine and placental surfaces along regions of epitheliochorial placentation in sheep. | Immunofluorescence | 19060096
 |
Mechanism(s) of increased vascular cell adhesion on nanostructured poly(lactic-co-glycolic acid) films.
Miller, Derick C, et al.
Journal of biomedical materials research. Part A, 73: 476-84 (2005)
2004
Afficher le résumé
Studies have shown that poly(lactic-co-glycolic acid) (PLGA) films with nanometer surface features promote vascular endothelial and smooth muscle cell adhesion. The objective of this in vitro research was to begin to understand the mechanisms behind this observed increase in vascular cell adhesion. Results provided evidence that nanostructured PLGA adsorbed significantly more vitronectin and fibronectin from serum compared to conventional (or those not possessing nanometer surface features) PLGA. When separately preadsorbing both vitronectin and fibronectin, increased vascular smooth muscle and endothelial cell density was observed on nanostructured (compared to conventional) PLGA. Additionally, blocking of cell-binding epitopes of fibronectin and vitronectin significantly decreased vascular cell adhesion on nanostructured (compared to conventional) PLGA. For this reason, results of the present in vitro study demonstrated that cell adhesive proteins adsorbed in different quantities and altered bioactivity on nanostructured compared to conventional PLGA topographies, which (at least in part) may account for the documented increased vascular cell adhesion on nanostructured PLGA. In this manner, this study continues to provide evidence for the promise of nanostructured PLGA in vascular tissue engineering applications. | | 15880725
 |
Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the alpha 5 beta 1 integrin
Orecchia, A. et al.
J. Cell Sci., 116:3479-3489 (2003)
2003
| | 12865438
 |
Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion.
Benjamin G Keselowsky, David M Collard, Andrés J García
Journal of biomedical materials research. Part A
66
247-59
2003
Afficher le résumé
Integrin-mediated cell adhesion to proteins adsorbed onto synthetic surfaces anchors cells and triggers signals that direct cell function. In the case of fibronectin (Fn), adsorption onto substrates of varying properties alters its conformation/structure and its ability to support cell adhesion. In the present study, self-assembled monolayers (SAMs) of alkanethiols on gold were used as model surfaces to investigate the effects of surface chemistry on Fn adsorption, integrin binding, and cell adhesion. SAMs presenting terminal CH(3), OH, COOH, and NH(2) functionalities modulated adsorbed Fn conformation as determined through differences in the binding affinities of monoclonal antibodies raised against the central cell-binding domain (OH > COOH = NH(2) > CH(3)). Binding of alpha(5)beta(1) integrin to adsorbed Fn was controlled by SAM surface chemistry in a manner consistent with antibody binding (OH > COOH = NH(2) > CH(3)), whereas alpha(V) integrin binding followed the trend: COOH >> OH = NH(2) = CH(3), demonstrating alpha(5)beta(1) integrin specificity for Fn adsorbed onto the NH(2) and OH SAMs. Cell adhesion strength to Fn-coated SAMs correlated with alpha(5)beta(1) integrin binding (OH > COOH = NH(2) > CH(3)), and experiments with function-perturbing antibodies demonstrated that this receptor provides the dominant adhesion mechanism in this cell model. This work establishes an experimental framework to analyze adhesive mechanisms controlling cell-surface interactions and provides a general strategy of surface-directed control of adsorbed protein activity to manipulate cell function in biomaterial and biotechnological applications. | | 12888994
 |
Molecular biology of fibronectin.
Hynes, R
Annu. Rev. Cell Biol., 1: 67-90 (1985)
1985
| | 3916323
 |
Serum spreading factor (vitronectin) is present at the cell surface and in tissues.
Hayman, E G, et al.
Proc. Natl. Acad. Sci. U.S.A., 80: 4003-7 (1983)
1982
Afficher le résumé
Monoclonal antibodies were prepared against a cell attachment-promoting protein, serum spreading factor, which had been partially purified from human serum by chromatography on glass bead columns. The antibodies selected were those that reacted with polypeptides that had cell attachment-promoting activity after sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Immunochromatography of human plasma on columns containing the monoclonal antibodies followed by affinity chromatography on heparin-Sepharose yielded material that in sodium dodecyl sulfate/polyacrylamide gel electrophoretic analysis gave polypeptides of molecular mass 65 and 75 kilodaltons. Both polypeptides bound each of three monoclonal antibodies and had cell attachment-promoting activity after transfer to nitrocellulose filters. Immunofluorescent staining of tissues with the monoclonal antibodies revealed a fibrillar pattern that was mostly associated with loose connective tissue and overlapped with fibronectin fibrils. Fetal membrane tissue, which showed strong staining with the antibodies in immunofluorescence, also gave 65- and 75-kilodalton polypeptides with cell attachment-promoting activity after chromatography on columns containing the monoclonal antibodies. One source of the tissue protein may be fibroblastic cells, because cultured human fibroblasts also stained with the monoclonal antibodies. The staining was fibrillar and appeared to be associated with the cell surface extracellular matrix. We propose the name "vitronectin" for the various forms of this protein, on the basis of its binding to glass and its adhesive properties. | | 6191326
 |
Fibronectin: purification, immunochemical properties, and biological activities.
Ruoslahti, E, et al.
Meth. Enzymol., 82 Pt A: 803-31 (1982)
1981
| | 7078457
 |