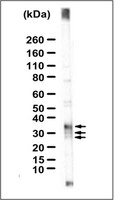
Assembled pre-B cell receptor complexes are retained in the endoplasmic reticulum by a mechanism that is not selective for the pseudo-light chain.
Brouns, GS; de Vries, E; Neefjes, JJ; Borst, J
The Journal of biological chemistry
271
19272-8
1996
Afficher le résumé
The pre-B cell receptor (BCR) complex, consisting of micro heavy chain, a pseudo-light chain, and the Mb-1/B29 heterodimer, directs the transition to the mature B cell stage. Plasma membrane expression of the pre-BCR is extremely low, despite its presumed signaling function. We have compared assembly and intracellular transport of the pre-BCR complex with that of the BCR complex in mature B cells. Synthesis and assembly rate of pre-BCR and BCR components are comparable. However, the pre-BCR is subject to a highly efficient retention mechanism, which only allows exit of a few percent of the complexes from the endoplasmic reticulum (ER). This small transported pool of pre-BCR complexes is significantly enriched for protein-tyrosine kinase activity, as compared with the ER-localized receptor pool. Accordingly, the Src-related tyrosine kinase Lyn was found in the transported glycoprotein fraction but not in association with ER-localized glycoproteins. Upon introduction of a conventional light chain into pre-B cells, plasma membrane receptor levels increased, but the efficiency of intracellular transport of the receptor complex was not restored to that in mature B cells. This indicates that the ER retention mechanism is not selective for the pseudo-light chain and may be inherent to pre-B cells. We propose that this retention mechanism contributes to the regulation of pre-BCR-mediated signal transduction. | 8702609
 |
Assembly and intracellular transport of the human B cell antigen receptor complex.
Brouns, GS; de Vries, E; Borst, J
International immunology
7
359-68
1994
Afficher le résumé
The B cell antigen receptor (BCR) complex consists of transmembrane (m) Ig, in non-covalent association with a disulphide-linked heterodimer of mb-1 and B29 gene products. The MB-1-B29 heterodimer is required for deposition of the BCR at the plasma membrane, as well as for coupling of the antigen receptor to intracellular signal transduction cascades. We have performed biosynthetic labelling studies using the mature B cell line Ramos to investigate the process of assembly of the BCR components. We conclude that association of the four components, Ig-heavy chain (HC) and -light chain (LC), MB-1 and B29, is required and sufficient to permit exit of the BCR complex out of the endoplasmic reticulum (ER). With the short pulse labelling procedures used, no evidence was found for transient participation of other molecules in complex formation. A 32 kDa glycoprotein was identified, which is serologically related to MB-1, but has a more acidic isoelectric point (pl) and a protein backbone of 21 kDa, as compared with 25 kDa for MB-1. This protein did not appear to participate in BCR complex formation and is most likely degraded prior to reaching the cis-Golgi. The MB-1 component was found to be the rate-limiting step in BCR complex formation, while Ig-HC, -LC and B29 are synthesized in excess. Ig-HC and -LC form disulphide-linked tetrameric complexes within 3 min after biosynthesis, with which B29 and MB-1 components associate independently, followed by disulphide bond formation between these heterodimeric partners. While partial BCR complexes containing B29 and mlg-H2L2 tetramers are rapidly formed and have a half-life of a few hours in the ER, entry of MB-1 into these complexes controls exit out of this compartment. | 7794818
 |